Abstract
In tissue engineering, it is well accepted that a scaffold surface has a decisive impact on cell behaviour. Here we focused on microglia—the resident immune cells of the central nervous system (CNS)—and on their response to poly(trimethylene carbonate-co-ε-caprolactone) (P(TMC-CL)) fibrous and flat surfaces obtained by electrospinning and solvent cast, respectively. This study aims to provide cues for the design of instructive surfaces that can contribute to the challenging process of CNS regeneration. Cell morphology was evidently affected by the substrate, mirroring the surface main features. Cells cultured on flat substrates presented a round shape, while cells with elongated processes were observed on the electrospun fibres. A higher concentration of the pro-inflammatory cytokine tumour necrosis factor-α was detected in culture media from microglia on fibres. Still, astrogliosis is not exacerbated when astrocytes are cultured in the presence of microglia-conditioned media obtained from cultures in contact with either substrate. Furthermore, a significant percentage of microglia was found to participate in the process of myelin phagocytosis, with the formation of multinucleated giant cells being observed only on films. Altogether, the results presented suggest that microglia in contact with the tested substrates may contribute to the regeneration process, putting forward P(TMC-CL) substrates as supporting matrices for nerve regeneration.
Keywords: electrospinning, microglia, scaffold surface, myelin, multinucleated giant cell, nerve tissue engineering
1. Introduction
Microglia, the resident immune cells of the central nervous system (CNS), play a key role in the maintenance of CNS homeostasis and in the management of tissue response to injury, although representing only approximately 10% of the total number of glial cells [1]. Microglia can secrete both pro-inflammatory cytokines that may lead to cell death, and anti-inflammatory molecules and neurotrophic factors that contribute to neuroprotection and regeneration [2]. Furthermore, in the context of an injury to the CNS, microglia are involved in the clearance of myelin debris that accumulates owing to Wallerian degeneration. This process is of paramount importance as the accumulation of debris has been associated with the inhibition of axonal regeneration [3]. Consequently, the diversity of microglia activities turn these cells into an interesting target for new therapies in the context of CNS regeneration [4].
It is now well established that topographic cues can have a considerable influence on cellular processes such as cell adhesion and differentiation (see [5,6] for a review). Under the scope of the development of scaffolds for CNS tissue engineering, neurons have been under the spotlight, so far. It has been shown that fibrous surfaces support axonal guidance and growth [7–9], as well as stem-cell differentiation into the neuronal lineage [10,11]. The number of studies investigating the influence of surface features on glial cells is increasing, yet focused on astrocytes, mainly due to astrocytes' key role in the formation of the glial scar in response to an injury to the CNS [12]. Astrocytes have been found to orientate their filamentous structure according to the features of the surface [13–16]. Moreover, although some authors did not find significant alterations in astrocyte activation when cells were seeded on a fibrous surface in comparison with cells cultured on flat solvent-cast films (assessed in terms of glial fibrillary acidic protein (GFAP) and vimentin protein expression) [14], others claimed that the contact of astrocytes with fibres is able to promote a decrease in GFAP expression [15] and an increase in glutamate uptake, contributing to neuroprotection in vivo [16]. Furthermore, by using micropatterned grooved scaffolds, mature astrocytes were found to revert into radial glia-like cells and consequently to a more pro-regenerative phenotype [17]. These studies highlight that by providing appropriate physical stimuli it is possible to bias the response of glial cells to injury.
Despite the important role ascribed to microglia, studies on microglia–material interaction are still in the infancy and have been focused on materials/structures for the design of implantable electrodes. The chemistry of the surface was found to influence the cytokine release profile of microglia depending on its hydrophobicity [18]. In what concerns surface topography, the effect of nanostructured silicone or poly(dimethylsiloxane) surfaces on microglia morphology, adhesion [19,20] or motility [19] was also investigated. More recently, it was demonstrated that microglia interact mechanically with silicone micropillars on a surface, and are affected by surface stiffness [21].
Foreseeing the design of a tissue engineering scaffold that could contribute to regeneration in the CNS, we explored the use of poly(trimethylene carbonate-co-ε-caprolactone) (P(TMC-CL)) to obtain matrices with different surface features. The preparation of fibres of this biodegradable polymer by electrospinning was previously reported [22], as were its remarkable properties in the context of tissue engineering for regeneration of the peripheral [23,24] and the central nervous system [25]. It is noteworthy that P(TMC-CL) has been shown to stimulate cortical neuron polarization and promote axonal elongation. Moreover, even in the presence of myelin, cortical neurons cultured on P(TMC-CL) films were found to extend more neurites, demonstrating the ability of P(TMC-CL) to tame myelin inhibition in a CNS lesion scenario [25]. Here we investigate the response of microglia to P(TMC-CL) surfaces prepared either by electrospinning or by solvent cast in order to gather important clues towards the design of instructive scaffolds that can contribute to the challenging process of CNS regeneration.
2. Material and methods
2.1. Polymer synthesis and characterization
The statistical P(TMC-CL) copolymer was prepared by ring-opening polymerization and subsequently purified as previously described [23]. The chemical composition of the purified copolymer was assessed by 1H nuclear magnetic resonance (NMR) and found to contain 11% mol of TMC, which was in accordance with the monomer ratio charged (10% mol TMC). The average number molecular weight and polydispersity index of the purified polymer were determined by size exclusion chromatography [22] and were found to be 8.2 × 104 and 1.61, respectively.
2.2. Substrate preparation
P(TMC-CL) fibres were prepared by electrospinning as previously described [22]. In brief, 10% (w/v) P(TMC-CL) solutions in dichloromethane (DCM; Merck, Germany) were dispensed at a controlled flow rate of 1 ml h−1 using a syringe pump (Ugo Basile, Italy). An electric field of 1 kV cm−1 was applied (Gamma High Voltage Research, Inc., FL, USA) between the spinneret (inner diameter 0.8 mm) and the flat collector (15 × 15 cm). Fibres were collected during 1–1.5 h onto 13 mm glass coverslips (Menzel-Glaser, Germany) distributed on top of aluminium foil.
P(TMC-CL) films were prepared by solvent casting as follows. A P(TMC-CL) solution in DCM (6% (w/v)) was cast onto a glass Petri dish. The solvent was left to evaporate overnight under a DCM-saturated atmosphere at room temperature (20–25°C).
After preparation, electrospun fibres and solvent-cast films were vacuum-dried for 24 h (vacuum oven; Raypa, Spain). Subsequently, 14 mm discs were punched out, packed under vacuum after an argon purge and sterilized by gamma irradiation (25 kGy, 60Co source).
2.3. Surface characterization
P(TMC-CL) samples were sputter-coated with gold–palladium for 90 s (SPI Supplies, PA, USA). Afterwards, the P(TMC-CL) surfaces were observed by scanning electron microscopy (SEM) using a Quanta 400FEG microscope (FEI, The Netherlands). The fibre diameter was quantified from SEM micrographs using image analysis software (ImageJ, v. 1.39; NIH, MD, USA). The fibre mean diameter and fibre diameter distribution were calculated from at least 100 measurements from three independent samples.
2.4. Primary cell isolation and culture
Primary cultures of microglia and astrocytes were obtained from postnatal (1–2 days) Wistar rat pups based on previously described procedures [26,27]. Briefly, pups were decapitated, the meninges were carefully stripped off and cortices dissected. Subsequently, the tissue was enzymatically digested using a papain solution (0.2 units ml−1; Sigma-Aldrich Química, Portugal) for 30 min at 37°C. The tissue was further dissociated using a pipette and, subsequently, plated into tissue culture treated flasks (Thermo Fisher Scientific, Portugal). The mixed glial cultures were maintained for 8–10 days at 37°C in high-glucose Dulbecco's modified Eagle medium (DMEM) supplemented with 10% (v/v) of heat-inactivated (56°C, 30 min) fetal bovine serum (FBS) and 1% (v/v) penicillin/streptomycin (P/S; 10 000 U ml−1 penicillin, 10 000 μg ml−1 streptomycin), all supplied by Gibco (Life Technologies S.A, Spain).
To obtain microglia, after culture confluence, the mixed glial cultures were shaken for 1 h using an orbital shaker (IKA, Germany) at 160 r.p.m. and 37°C. The supernatant enriched with microglia was collected and centrifuged (200g, 5 min). Microglial cell culture purity was quantified after immunolabelling using CD11b antibody (1 : 200; Abcam, Belgium) and found to be above 90% (see details in the electronic supplementary material) in accordance with previous reports that used a similar isolation technique [18,28]. A total of 6 × 104 viable cells ml−1 were seeded on P(TMC-CL) substrates secured at the bottom of a 24-well plate with a silicone o-ring (Epidor, Spain) and cultured in DMEM/F12 medium (Gibco) supplemented with 10% FBS and 1% P/S for 1 or 5 days. Glass coverslips were used as the experimental control.
After collecting microglia, mixed glial cultures were shaken for an additional 22 h to remove oligodendrocytes. The remaining cell layer, mainly composed of astrocytes, was maintained in culture in supplemented DMEM.
2.5. Cytoskeleton immunolabelling
To analyse cell morphology, cells were fixed with paraformaldehyde (4% (w/v), in phosphate-buffered saline; PBS) and immunostained for F-actin as follows. Cell external fluorescence was quenched using 50 mM NH4Cl (Merck) for 10 min. After washing with PBS (three times, 5 min) cells were permeabilized with 0.1% (v/v) Triton X-100 (in PBS) for 5 min. Afterwards, cells were washed with PBS, incubated with 5% (w/v) bovine serum albumin (BSA; Merck) in PBS for 30 min and, thereafter, incubated with Alexa Fluor 488 phalloidin (1 : 40; Invitrogen, Life Technologies). Subsequently, cells were washed with PBS and stained with 4′,6-diamidino-2-phenylindole (DAPI, 0.1 μg ml−1 in PBS; Sigma-Aldrich). Samples were observed under an inverted fluorescence microscope (Axiovert 200; Zeiss, Germany) or confocal microscope (Leica Microsystems, Germany).
2.6. Microglia morphology analysis
Microglia morphology was analysed using the Fraclac plug-in for ImageJ. The box counting fractal dimension (DB) [29] as well as morphometrics based on the convex hull were calculated. Images of the cytoskeleton (F-actin) of individual cells (n = 50) were applied, after conversion to binary images and manually outlining the cell contour. The morphometric parameters calculated include area and circularity, and the results are presented in pixels.
2.7. Cytokine quantification
At the defined time point, cell culture supernatants from microglia seeded on different P(TMC-CL) substrates were collected and, after centrifugation (16 000g, 4°C, 10 min) to remove cell debris, stored at −20°C for posterior analysis. Cell culture media from cells activated with lipopolysaccharide (LPS, 100 ng ml−1, 3 h; Sigma-Aldrich) was also analysed to serve as the positive control for microglia activation [30].
Tumour necrosis factor-α (TNFα; RayBiotech, GA, USA) and interleukin-6 (IL-6), interferon-γ (INFγ) and interleukin-10 (IL-10; all supplied by Biolegend, CA, USA) were quantified from microglia culture supernatants by enzyme-linked immunosorbent assay (ELISA) following the manufacturer's instructions.
2.8. Myelin phagocytosis assay
Myelin phagocytosis by microglia when seeded on different substrates was evaluated as follows. Rat brain myelin was obtained as previously described [31]. Five days after microglia seeding, a myelin suspension was added to the cell culture media to a final concentration of 2.5 µg ml−1 [32]. After 24 h in contact with myelin, cells were washed, stained for CD11b and subsequently fixed and permeabilized as described above. Cells were counterstained using myelin-binding protein (MBP) antibody (1 : 200; Chemicon, Millipore, MA, USA) at 4°C, overnight followed by 1 h of incubation with Alexa Fluor 488 donkey anti-rat IgG (1 : 1000; Invitrogen, Life Technologies). DAPI was applied to label cell nuclei. Cultures were observed using an inverted fluorescence microscope (Axiovert; Zeiss). Cells containing MBP (measure for myelin ingestion) and multinucleated giant cells (MGCs) were quantified from three different experiments and data are presented relative to the total number of cells.
2.9. Effect of microglia-conditioned media on astrocyte metabolic activity and gene expression
Astrocytes (4 × 104 viable cells ml−1; passage 4–7) were seeded on 24-well plates using supplemented DMEM. After adhesion overnight, the cell culture medium was changed to microglia-conditioned media collected after 5 days in contact with P(TMC-CL) substrates. As a control condition (non-treated cells), astrocyte cultures were conducted in supplemented DMEM/F12 (microglia culture medium; see §2.4).
Cell metabolic activity was assessed after 24 and 72 h by two different methods. Cellular ATP content was measured using Celltiter-Glo (Promega, WI, USA), following the manufacturer's instructions. To assess resazurin metabolization, cells were incubated (4 h, 37°C) with a resazurin (Sigma-Aldrich) solution (0.1 mg ml−1, in PBS) and the fluorescence (λex = 530 nm, λem = 590 nm) in the cell culture medium was measured (Synergy Mx; Biotek, Portugal).
Expression of genes related to astrogliosis, namely GFAP, collagen IV and vimentin, was assessed. Cell lysis and RNA purification were performed using Quick-RNA MiniPrep from Zymo Research (CA, USA), according to the manufacturer's instructions. Reverse transcription was done with SuperScript III (Invitrogen). Primer sequences are provided in the electronic supplementary material. Hypoxanthine-guanine phosphoribosyltransferase (Hprt) was applied as the reference gene. Polymerase chain reaction (PCR) was performed using HotStarTaq DNA polymerase (Qiagen, USA) for 34 cycles. Quantification of band intensity was done using ImageLab software, version 3.0 (Bio-Rad, Portugal).
To quantify GFAP expression at the protein level, the protein was assessed in astrocyte cultures by immunocytochemistry. In brief, astrocytes treated with microglia-conditioned media were fixed using paraformaldehyde and fluorescently labelled using the anti-GFAP antibody (rabbit anti-GFAP, 1 : 500; Dako), according to the procedure described in §2.4. Images were acquired applying constant fluorescence intensity. At least 25 images for each treatment from two independent experiments were quantified using ImageJ software.
2.10. Statistical analysis
Statistical analysis was performed using prism 5.0 software (GraphPad, CA, USA). A parametric t-test was applied to assess differences in cell morphology parameters and GFAP expression. Statistical differences between groups for cytokine concentration, astrogliosis markers and myelin phagocytosis were calculated by applying the non-parametric Mann–Whitney test. A p-value lower than 0.05 was considered statistically significant and is denoted by * or *** if p < 0.001.
3. Results
3.1. Substrate characterization
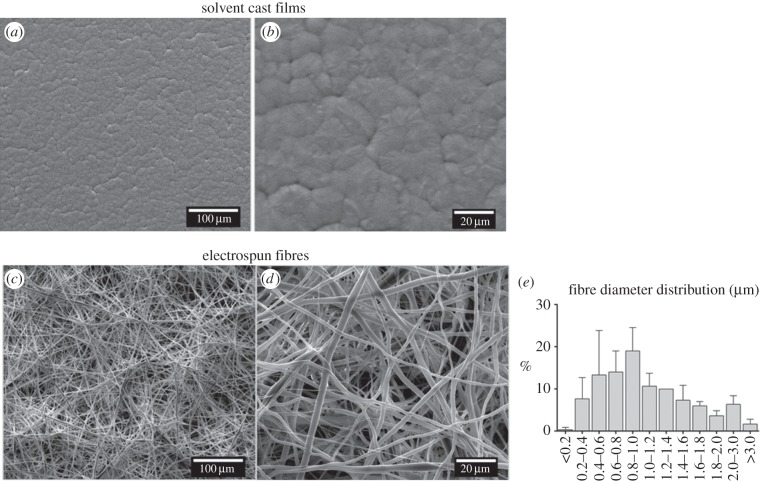
By using different processing techniques (electrospinning and solvent casting), distinctive P(TMC-CL) surfaces were obtained, as observed in the representative SEM micrographs presented in figure 1. Solvent-cast films show a spherulitic morphology (figure 1a,b) characteristic of a semicrystalline material [33]. The preparation of P(TMC-CL) fibres by electrospinning was previously optimized [22]. Under the conditions selected for the present study, the prepared electrospun membranes show a typical fibrous and randomly oriented structure (figure 1c,d). Bead defects are not observed. The mean fibre diameter was determined to be 1.09 ± 0.1 μm, being the fibre diameter distribution as depicted in figure 1e.
Figure 1.
Scanning electron microscopy (SEM) photomicrographs of the prepared P(TMC-CL) surfaces. (a,b) Films obtained by solvent casting and (c,d) fibres obtained by electrospinning. (e) Fibre diameter distribution as calculated from 100 measurements from three independent samples (average ± s.d.).
3.2. Effect of P(TMC-CL) surfaces on microglia
3.2.1. Microglia morphology
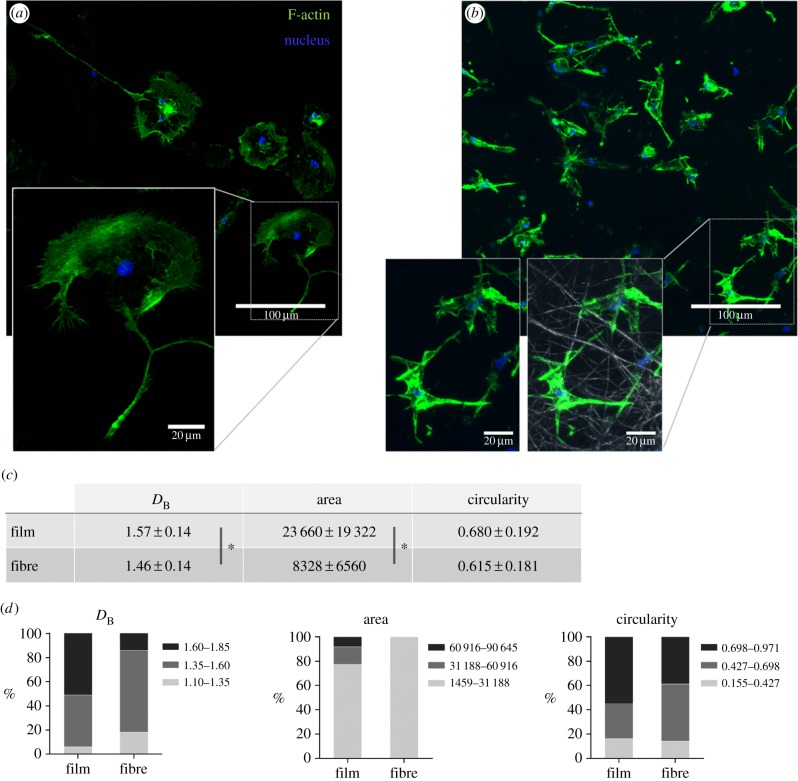
The morphology of microglia cells when seeded on different P(TMC-CL) surfaces was analysed after immunolabelling of F-actin. Cell cytoskeleton organization was found to be significantly affected by the surface features, as can be observed in figure 2. On P(TMC-CL) films, microglia present a round shape and long protrusions (figure 2a). Conversely, microglia seeded on P(TMC-CL) electrospun fibres show a smaller and more elongated cytoplasm (figure 2b), with actin concentrated at the points of cell adhesion along the fibre (figure 2b). Image analysis shows that microglia seeded on P(TMC-CL) films has an increased complexity compared with cells cultured on fibres, as indicated by the higher box counting fractal dimension DB [29]. Moreover, cell area was also found to be significantly increased on microglia seeded on P(TMC-CL) films (figure 2c). Although no statistical differences were found comparing mean values of circularity, it can be observed from the graphs representing the tercile distribution of the data (division of the distribution into three parts, each containing one-third of the population) that on P(TMC-CL) films a higher percentage of cells show a circularity close to 1—the theoretical circularity of a circle (figure 2d).
Figure 2.
Microglia morphology when cultured (5 days) on P(TMC-CL) substrates. (a,b) Confocal Z-projection images of F-actin and cell nuclei of microglia seeded on P(TMC-CL) (a) films or (b) fibres. In the presented detail of (b) the fibrous structure of the electrospun mat (grey) is also shown. (c,d) Characterization of microglia morphology by image analysis using the box counting fractal dimension (DB) and morphometrics based on a convex hull (n = 50). (c) Average ± standard deviation values for the morphological parameters investigated: DB, cell area and circularity. (d) Graphical representation of morphological parameters divided in terciles. Asterisks (*) denote statistical significance, p < 0.05.
3.2.2. Cytokine release profile
Variations on microglia morphology have been traditionally associated with distinct functional states [34,35]. Therefore, to evaluate whether the differences found on microglia morphology, when seeded on the different P(TMC-CL) surfaces, can lead to alterations in cytokine release profile, IL-10 and TNFα were quantified in the cell culture medium at day 1 of culture and IL-10, TNFα, IL-6 and INFγ at day 5 of culture (figure 3). Although the differences did not achieve statistical significance, a higher concentration of the anti-inflammatory cytokine IL-10 was detected on the cell culture medium from microglia cultured on P(TMC-CL) fibres than on the medium obtained from cells seeded on solvent-cast films (figure 3a,b). Additionally, TNFα was found to be increased in the cell culture medium of cells adhered to the P(TMC-CL) fibrous surface, as compared with cells adhered on solvent-cast films; this difference was statistically significant at day 1 of culture (figure 3a). At day 5 of culture, the concentration of both TNFα and IL-6 tend to be increased, not statistically significant though (figure 3c). It is worthwhile mentioning that a sharp increase in TNFα and IL-6 concentration was observed when microglia were stimulated with LPS (figure 3c). The concentration of INFγ was found to be low and close to the detection limit of the ELISA performed (3.2 pg ml−1), resulting in no differences between samples (data not shown).
Figure 3.
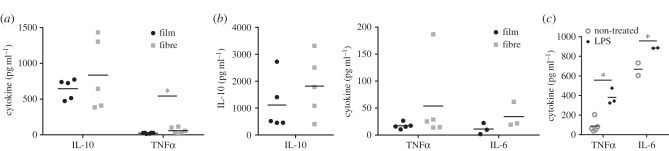
Cytokine release by microglia cultured on P(TMC-CL) substrates. Dot-plot showing the concentration of (a) IL-10 and TNFα in cell culture media after 1 or (b) 5 days in culture when seeded on P(TMC-CL) surfaces (n = 5). Panel (b) also shows the concentration of IL-6 (n = 3). (c) Release of pro-inflammatory cytokines (TNFα, IL-6) after microglia stimulation with lipopolysacharide (100 ng ml−1, 3 h, LPS). Asterisks (*) denote statistical significance, p < 0.05.
Analysing the concentration of cytokines in the cell culture media over time, it can be observed that when cells were cultured in contact with P(TMC-CL) fibres the IL-10 concentration tended to increase, whereas the TNFα concentration was maintained. In the case of cells cultured on P(TMC-CL) films, no alteration in cytokine concentration was detected between the two time points analysed (figure 3).
3.2.3. Myelin phagocytosis
One of the key functions of microglia in the aftermath of a lesion to the CNS is the clearance of myelin debris since myelin accumulation exposes inhibitory molecules converting the lesion region into a non-permissive substrate for axonal regrowth [3]. To investigate whether the surface can influence the ability of microglia to phagocytose myelin, myelin was added in suspension to cells cultured on the different substrates and the percentage of cells engulfing myelin was quantified after immunolabelling.
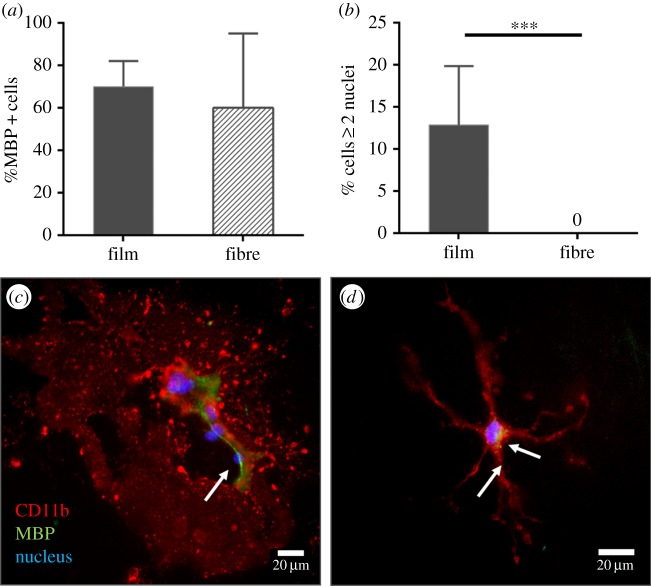
The overall percentage of microglia found to engulf myelin was greater than 60% for cells cultured both on P(TMC-CL) films and on P(TMC-CL) fibres, with this parameter tending to be higher for cells seeded on films (figure 4a). As previously mentioned, cell morphology is markedly influenced by the P(TMC-CL) surface. Figure 4 shows that it is further affected by the presence of myelin. The round cells with long protrusions found on P(TMC-CL) films (figure 2) were able to form MGCs when in contact with myelin (figure 4b,c). On the other hand, in the microglia cultures performed in contact with fibrous substrates, MGCs were not observed (figure 4b). Conversely, cells tend to increase the number of ramifications (figure 4d).
Figure 4.
Myelin phagocytosis assay. (a) Quantification of the percentage of microglia cells that co-localize with myelin-binding protein (MBP). (b) Percentage of multinucleated giant cells found in microglia cultures seeded on P(TMC-CL) films, or fibres in the presence of myelin. Bars represent mean values and error bars show standard deviation (n = 3). Asterisks (***) denote statistical significance, p < 0.001. (c,d) Fluorescence microscopy images of microglia cultured on P(TMC-CL) (c) films and (d) fibres when in contact with myelin. Arrows indicate myelin inside the cells.
3.3. Effect of microglia-conditioned media on primary astrocyte cultures
3.3.1. Astrocyte metabolic activity
The increase in astrocyte proliferation is one of the events associated with reactive astrogliosis, which is widely used as a pathological hallmark of the injured CNS [36]. Microglia cells are the immune regulators of astrogliosis [37], namely by releasing a variety of cytokines [36]. To understand whether microglia cells cultured on different (TMC-CL) surfaces can release factors with an impact on astrocytes, astrocyte metabolic activity was assessed after being cultured with microglia-conditioned media. Measures of metabolic activity were taken to be indicative of cell proliferation.
The cell metabolic activity of astrocytes when in contact with microglia-conditioned media showed a tendency to increase compared with the cell metabolic activity of non-treated cells (figure 5a,b). Conditioned media obtained from microglia cultures on P(TMC-CL) fibres or solvent-cast films were found to have a similar effect on astrocyte metabolic activity (figure 5a,b). Comparable results were obtained when astrocyte metabolic activity was assessed after 72 h in contact with microglia-conditioned media (data not shown). Figure 5c shows the typical morphology [38] of the astrocytic cell culture. No alterations were identified after incubating astrocytes with the different microglia-conditioned media under investigation.
Figure 5.
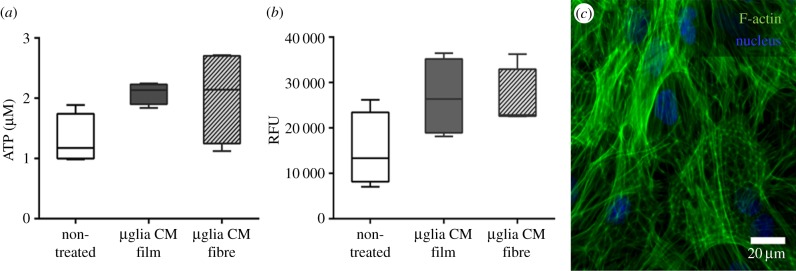
Astrocyte proliferation when in contact with microglia-conditioned media. Box-whisker plots (n = 4) showing (a) ATP production and (b) resazurin metabolism by astrocytes when in contact with microglia-conditioned media (µglia CM) during 24 h. The medium was recovered from microglial cultures after 5 days in contact with P(TMC-CL) films or fibres. Non-treated cells were maintained in supplemented DMEM/F12 media. (c) F-actin labelling of astrocytes incubated with microglia-conditioned media obtained from cultures on fibrous meshes.
3.3.2. Expression of astrogliosis markers
Astrogliosis has been associated with the upregulation of some genes, namely GFAP and vimentin [12]. Collagen type IV is the main constituent of the glial scar and its expression is increased in astrocytes in response to injury [39].
Astrocyte expression of astrogliosis gene markers was found not to be significantly affected by microglia-conditioned media in comparison with non-treated cells (figure 6a). Additionally, the P(TMC-CL) surface on which microglia were cultured did not show an effect on GFAP, vimentin or collagen type IV gene expression.
Figure 6.
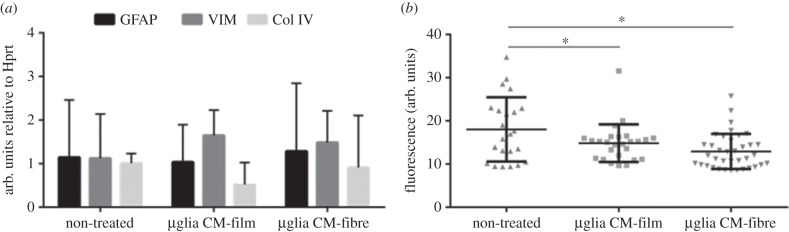
Expression of astrogliosis markers. (a) mRNA expression of glial fibrillary acidic protein (GFAP), vimentin (VIM) and collagen type IV (Col IV) in astrocytes treated with conditioned media obtained from microglia seeded on P(TMC-CL) solvent-cast films, or electrospun fibres, after 5 days in culture. Non-treated cells were maintained in supplemented DMEM/F12. Bars represent mean values and error bars show standard deviation (n = 4). (b) Fluorescence intensity of GFAP labelling in astrocytes cultured with microglia-conditioned media. Images were acquired with constant fluorescence intensity. At least 25 images were quantified from two independent experiments. Asterisks (*) denote statistical significance, p < 0.05.
The expression of GFAP was assessed at the protein level by quantification of the fluorescence after GFAP immunolabelling. When using conditioned media from microglia cultured on P(TMC-CL) surfaces GFAP fluorescent labelling were decreased (figure 6b) compared with non-treated cells, suggesting that the factors released by microglia cultured on these surfaces do not activate astrocytes (figure 6b). Microglia-conditioned media obtained from cells seeded on glass coverslips was herein applied as an experimental control, leading to an increase in GFAP protein expression and confirming cell responsiveness in the experimental set-up applied (data not shown).
4. Discussion
In the past few years, the understanding of the role of surface topographic features has gained substantial relevance in the context of the design of tissue engineering scaffolds for nerve regeneration. The focus was initially directed to neuronal cells [7,9,11] but more recent studies are contributing to shed some light on the effect of this parameter on other CNS cellular key players, such as astrocytes [14–17]. It is known that microglia, the immune cells of the CNS, play a critical role in CNS homeostasis as well as being in the frontline of the tissue response to injury [1]. Particularly, microglia cells can release cytokines and other molecules, activating cells at the lesion site, recruiting others and modulating their own function in an autocrine effect [37]. However, taking the role of microglia in a lesion scenario into consideration, the impact of the surface properties, in particular of topographical features, on the microglia response has been overlooked at large. This was the main goal of this study.
As previously reported for other cell types [6], in this work it was shown that microglia organize their cytoskeleton according to the features of the surface to which the cell adhere. On P(TMC-CL) solvent-cast films, microglia present a rounder shape and long protrusions, whereas on fibres the cell cytoskeleton elongates along the fibre direction and the cell area is smaller. Variations on microglia cell shape have been commonly taken as an indication of distinct functional states. Classically, amoeboid features have been associated with increased phagocytic and pro-inflammatory activities of microglia, whereas a ramified morphology suggests a quiescent state [34,35]. This classification is currently issue of active debate, as some reports show that different microglia activation states do not require alterations in cell morphology [40]. Still, the morphological features and functional state of microglia are correlated in different recent studies [41–43]. Here, the major differences in the morphology of microglia seeded on P(TMC-CL) substrates are reported. Although cell morphology was found to be neither marked amoeboid nor strictly ramified, it was observed that cells cultured on P(TMC-CL) films are larger (increased area) and tend to present an increased circularity. This cell shape could be considered an indication of a more pro-inflammatory profile. However, in regard to the cytokine release, it was found that microglia seeded on P(TMC-CL) films release less TNFα to the cell culture medium than cells adhered to fibres, at day 1 of culture. Extending the period of culture of microglia on the tested substrates resulted in no statistical differences in the release of both pro-inflammatory (IL-6 and TNFα) and anti-inflammatory (IL-10) cytokines. These results indicate that P(TMC-CL) surface features are determinant for microglia shape, but this does not impact long-term cytokine release, suggesting that cell shape is not a parameter on which one can directly predict functional state. A similar issue has been previously raised by Bartneck et al. [44] when comparing macrophages cultured on flat spin-coated films and hydrogel-coated nanofibres with variable fibre density (and pore size). The authors claimed that the effect on cell morphology and the expression of surface markers is strongly affected by the biomaterial to which cells adhere. The analysis of microglia morphology using box counting analysis can bring new insights into this topic. The presented results show that cells seeded on P(TMC-CL) films have a higher complexity than cells cultured on fibres, as measured by the DB parameter. It has been proposed that microglia in the resting state have an increased complexity [29]. Taken together, our data suggest that microglia cultured on the P(TMC-CL) films are in a less activated state than cells cultured on fibres.
Interestingly, the effect of the surface features on cytokine release by primary microglia reported here shows a different trend compared to that described for macrophages. Previous studies using poly(l-lactide) [45] or poly(ε-caprolactone) [46] demonstrated that the concentration of pro-inflammatory molecules is lower in cultures in contact with electrospun fibrous surfaces than in cells on solvent-cast films. A surface topography that induces macrophage elongation was found to favour macrophage polarization into an anti-inflammatory phenotype, and, although the mechanisms are still not fully described, it was suggested that polarization via topographic signalling is mediated by actin cytoskeleton contractility [47]. The differences found in this study on microglia behaviour highlight the need for studying microglia in detail. Even though sharing relevant lineage features with macrophages, these cells can react differently to stimuli, as previously reported when testing different chemical factors [32,48].
In the context of an injury to the CNS, the contribution of microglia to the clearance of debris is of primary importance, as inefficient removal of myelin debris is associated with the inhibition of nerve regeneration [3]. It has been demonstrated that myelin phagocytosis is affected by the stimulation of microglia with different cytokines [32]. Thus, in the present work it was investigated whether culturing cells on different P(TMC-CL) surfaces can have an impact on microglia-mediated phagocytosis. A previous report showed that microglia in basal conditions or stimulated with anti-inflammatory cytokines (IL-4 and IL-13) were more efficient in myelin phagocytosis, with 70–75% of these cells being able to incorporate myelin in a phagocytosis assay. Conversely, less than 50% of the cells engulfed myelin if stimulated with LPS and INFγ [32]. In the context of Alzheimer's disease, it has been demonstrated that the accumulation of pro-inflammatory molecules such as LPS, IL-1β or β-amyloid fibrils induces microglia dysfunction, limiting their phagocytic activity [49]. In this study, the percentage of cells that engulfed myelin was found to be above 60% for cultures conducted either on P(TMC-CL) solvent-cast films or on fibrous membranes. This result suggests that the P(TMC-CL) surfaces provide physical and/or chemical cues that promote phagocytosis without the need for additional chemical stimuli and may actively contribute to the establishment of a pro-regenerative environment.
Despite the fact that the percentage of cells that engulfed myelin was found not to be influenced by the surface, a remarkable difference was observed in microglia when in the presence of myelin. More than 10% of microglia seeded on P(TMC-CL) films were found to form MGCs, a phenomenon that was not observed when cells were adhered to fibres (0%). Although the morphology of microglia cells was affected by the surface features as described above, the formation of MGCs was clearly a consequence of the presence of myelin, as this event was not detected in its absence. The role of MGCs derived from microglia has been poorly discussed in the open literature. These cells have been found to accumulate with age [50], and are also associated with some neuropathologies, namely HIV-related dementia [51]. Microglia activation to form MGCs can be triggered by inflammatory cytokines [41,52,53] as well as in response to phagocytosis of cell debris [52,54]. MGCs have an increased phagocytic activity [52,54], which may represent an advantage when large amounts of debris accumulate due to Wallerian degeneration. To the best of our knowledge this is the first study that analyses the effect of the biomaterial surface on microglia in light of MGC formation. A recent publication using monocyte-derived macrophages demonstrates that orthogonal features on chitosan scaffolds favoured macrophage fusion and MGC formation, comparing with a diagonal architecture [55]. Nonetheless, the authors were able to correlate this effect with the increase in TNFα in the cell culture media. In this study, the concentration of TNFα when cells were seeded on P(TMC-CL) films was found to be low, suggesting that this cytokine was not involved in the stimulation of MGC formation. It cannot be excluded that the concentration of TNFα was altered in the presence of myelin, but, if this was the case, it remains to be clarified why this was only in cells seeded on P(TMC-CL) films. In this context, the obtained results point out the importance of the substrate influencing directly the formation of MGCs. In our interpretation of the obtained results, the surface provided by electrospun fibres may be hampering cytoskeleton re-arrangement, cytoplasm enlargement and cell fusion, compromising, therefore, the formation of MGCs in comparison with what occurs on solvent-cast films.
There is increasing evidence that a reciprocal modulation between microglia and astrocytes takes place after CNS injury [56]. Microglia are the first cells arriving at the lesion site and the cytokines released by these cells, namely TNFα and IL-1β, can induce astrocyte proliferation, influencing the glial scar formation [57]. On the other hand, molecules produced by astrocytes are believed to modulate microglia activation in the chronic phase of injury [56]. Taking these aspects into consideration, in this study it was investigated how the response of microglia to different surfaces can influence astrocyte activation markers. It was found that none of astrogliosis markers analysed are upregulated when astrocytes are treated with conditioned media from microglia cultured on P(TMC-CL) substrates. A previous study reported no activation of astrogliosis markers in astrocytes cultured with conditioned medium from resting microglia [58]. Therefore, our results point to the fact that the amount of pro-inflammatory cytokines produced by cells when seeded on P(TMC-CL) substrates does not trigger a significant activation of microglia that could, consequently, have an impact on astrocyte activation.
5. Conclusion
This work describes the effect of different substrates—P(TMC-CL) fibres and flat films—on primary microglia cells. Overall, the results presented show that both surfaces provide cues that may guide microglia towards a pro-regenerative profile, while evident differences were found on cell morphology, in line with the topographical features of the surface. Accordingly, it was pointed out that, when different surfaces are under investigation, microglia behaviour cannot be anticipated from cellular shape. Although the TNFα concentration was found to be increased in the early response to fibrous substrates, overall, the factors released by the cells were not able to trigger astrogliosis, independent of the surface. It is noteworthy that a significant percentage of microglia seeded on P(TMC-CL) substrates was found to participate in the phagocytosis of myelin. Moreover, only cells seeded on P(TMC-CL) flat films were able to form MGCs, pointing to the decisive role of the surface on the response of microglia to myelin debris. These results, along with our previous findings reporting that P(TMC-CL) has suitable properties for neuronal growth [24,25], put forward P(TMC-CL) as a supportive material for tissue regeneration in the context of an injury of the CNS.
Supplementary Material
Supplementary Material
Acknowledgements
The authors acknowledge the Centro de Materiais da Universidade do Porto (CEMUP; REEQ/1062/CTM/2005 from FCT) for SEM and 1H-NMR analyses. The authors wish to thank Renato Socodato for the fruitful discussions.
Ethics statement
All experiments involving animals and their care were conducted in compliance with institutional ethics guidelines and with the approval of Portuguese Veterinary Authorities—Direcção-Geral de Alimentação e Veterinária (DGAV).
Funding statement
This work was financed by FEDER funds through the Programa Operacional Factores de Competitividade—COMPETE and by Portuguese funds through FCT—Fundação para a Ciência e a Tecnologia in the framework of the project PEst-C/SAU/LA0002/2011. L.P. and D.R. thank FCT for their PhD grants (SFRH/BD/46015/2008 and SFRH/BD/64079/2009).
References
- 1.Aguzzi A, Barres BA, Bennett ML. 2013. Microglia: scapegoat, saboteur, or something else? Science 339, 156–161. ( 10.1126/science.1227901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.David S, Kroner A. 2011. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 12, 388–399. ( 10.1038/nrn3053) [DOI] [PubMed] [Google Scholar]
- 3.Schwab JM, Brechtel K, Mueller CA, Failli V, Kaps HP, Tuli SK, Schluesener HJ. 2006. Experimental strategies to promote spinal cord regeneration—an integrative perspective. Prog. Neurobiol. 78, 91–116. ( 10.1016/j.pneurobio.2005.12.004) [DOI] [PubMed] [Google Scholar]
- 4.Hernandez-Ontiveros DG, Tajiri N, Acosta S, Giunta B, Tan J, Borlongan CV. 2013. Microglia activation as a biomarker for traumatic brain injury. Front. Neurol. 26, 30 ( 10.3389/fneur.2013.00030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtis A, Wilkinson C. 1997. Topographical control of cells. Biomaterials 18, 1573–1583. [DOI] [PubMed] [Google Scholar]
- 6.Martínez E, Engel E, Planell JA, Samitier J. 2009. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann. Anat. 191, 126–135. [DOI] [PubMed] [Google Scholar]
- 7.Liu T, Houle JD, Xu J, Chan BP, Chew SY. 2012. Nanofibrous collagen nerve conduits for spinal cord repair. Tissue Eng. A 18, 1057–1066. ( 10.1089/ten.tea.2011.0430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nisbet DR, Rodda AE, Horne MK, Forsythe JS, Finkelstein DI. 2009. Neurite infiltration and cellular response to electrospun polycaprolactone scaffolds implanted into the brain. Biomaterials 30, 4573–4580. ( 10.1016/j.biomaterials.2009.05.011) [DOI] [PubMed] [Google Scholar]
- 9.Yucel D, Kose GT, Hasirci V. 2010. Polyester based nerve guidance conduit design. Biomaterials 31, 1596–1603. ( 10.1016/j.biomaterials.2009.11.013) [DOI] [PubMed] [Google Scholar]
- 10.Xie J, Willerth SM, Li X, Macewan MR, Rader A, Sakiyama-Elbert SE, Xia Y. 2009. The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials 30, 354–362. ( 10.1016/j.biomaterials.2008.09.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim SH, Liu XY, Song H, Yarema KJ, Mao HQ. 2010. The effect of nanofiber-guided cell alignment on the preferential differentiation of neural stem cells. Biomaterials 31, 9031–9039. ( 10.1016/j.biomaterials.2010.08.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pekny M, Nilsson M. 2005. Astrocyte activation and reactive gliosis. Glia 50, 427–434. ( 10.1002/glia.20207) [DOI] [PubMed] [Google Scholar]
- 13.Chow WN, Simpson DG, Bigbee JW, Colello RJ. 2007. Evaluating neuronal and glial growth on electrospun polarized matrices: bridging the gap in percussive spinal cord injuries. Neuron Glia Biol. 3, 119–126. ( 10.1017/S1740925X07000580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao H, Marcy G, Goh ELK, Wang F, Wang J, Chew SY. 2012. The effects of nanofiber topography on astrocyte behavior and gene silencing efficiency. Macromol. Biosci. 12, 666–674. ( 10.1002/mabi.201100436) [DOI] [PubMed] [Google Scholar]
- 15.Min SK, Kim SH, Kim CR, Paik SM, Jung SM, Shin HS. 2013. Effect of topography of an electrospun nanofiber on modulation of activity of primary rat astrocytes. Neurosci. Lett. 534, 80–84. ( 10.1016/j.neulet.2012.11.015) [DOI] [PubMed] [Google Scholar]
- 16.Zuidema JM, Hyzinski-García MC, Van Vlasselaer K, Zaccor NW, Plopper GE, Mongin AA, Gilbert RJ. 2014. Enhanced GLT-1 mediated glutamate uptake and migration of primary astrocytes directed by fibronectin-coated electrospun poly-l-lactic acid fibers. Biomaterials 35, 1439–1449. ( 10.1016/j.biomaterials.2013.10.079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattotti M, Alvarez Z, Ortega JA, Planell JA, Engel E, Alcántara S. 2012. Inducing functional radial glia-like progenitors from cortical astrocyte cultures using micropatterned PMMA. Biomaterials 33, 1759–1770. ( 10.1016/j.biomaterials.2011.10.086) [DOI] [PubMed] [Google Scholar]
- 18.Leung BK, Biran R, Underwood CJ, Tresco PA. 2008. Characterization of microglial attachment and cytokine release on biomaterials of differing surface chemistry. Biomaterials 29, 3289–3297. ( 10.1016/j.biomaterials.2008.03.045) [DOI] [PubMed] [Google Scholar]
- 19.Amadio S, De Ninno A, Montilli C, Businaro L, Gerardino A, Volonté C. 2013. Plasticity of primary microglia on micropatterned geometries and spontaneous long-distance migration in microfluidic channels. BMC Neurosci. 14, 121 ( 10.1186/1471-2202-14-121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persheyev S, Fan Y, Irving A, Rose MJ. 2011. BV-2 microglial cells sense micro-nanotextured silicon surface topology. J. Biomed. Mater. Res. A 99 A, 135–140. ( 10.1002/jbm.a.33159) [DOI] [PubMed] [Google Scholar]
- 21.Minev IR, Moshayedi P, Fawcett JW, Lacour SP. 2013. Interaction of glia with a compliant, microstructured silicone surface. Acta Biomater. 9, 6936–6942. ( 10.1016/j.actbio.2013.02.048) [DOI] [PubMed] [Google Scholar]
- 22.Pires LR, Guarino V, Oliveira MJ, Ribeiro CC, Barbosa MA, Ambrosio L, Pêgo AP. In press. Ibuprofen-loaded poly(trimethylene carbonate-co-ε-caprolactone) electrospun fibers for nerve regeneration. J. Tissue Eng. Regen. Med. ( 10.1002/term.1792) [DOI] [PubMed] [Google Scholar]
- 23.Pêgo AP, Poot AA, Grijpma DW, Feijen J. 2001. Copolymers of trimethylene carbonate and epsilon-caprolactone for porous nerve guides: synthesis and properties. J. Biomater. Sci. Polym. Ed. 12, 35–53. ( 10.1163/156856201744434) [DOI] [PubMed] [Google Scholar]
- 24.Pêgo AP, Poot AA, Grijpma DW, Feijen J. 2003. Biodegradable elastomeric scaffolds for soft tissue engineering. J. Control Release 87, 69–79. ( 10.1016/S0168-3659(02)) [DOI] [PubMed] [Google Scholar]
- 25.Rocha DN, Brites P, Fonseca C, Pêgo AP. 2014. Poly(trimethylene carbonate-co-ε-caprolactone) promotes axonal growth. PLoS ONE 9, e88593 ( 10.1371/journal.pone.0088593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarthy K, de Vellis J. 1980. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 85, 890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barateiro A, Miron VE, Santos SD, Relvas JB, Fernandes A, Ffrench-Constant C, Brites D. 2012. Unconjugated bilirubin restricts oligodendrocyte differentiation and axonal myelination. Mol. Neurobiol. 47, 632–644. ( 10.1007/s12035-012-8364-8) [DOI] [PubMed] [Google Scholar]
- 28.Cristóvão AC, Saavedra A, Fonseca CP, Campos F, Duarte EP, Baltazar G. 2010. Microglia of rat ventral midbrain recovers its resting state over time in vitro: let microglia rest before work. J. Neurosci. Res. 88, 552–562. ( 10.1002/jnr.22219) [DOI] [PubMed] [Google Scholar]
- 29.Karperien A, Ahammer H, Jelinek HF. 2013. Quantitating the subtleties of microglial morphology with fractal analysis. Front. Cell. Neurosci. 7, 1–34. ( 10.3389/fncel.2013.00003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang Y, Lee J, Hsieh C, Hsiao G, Chou D, Sheu J. 2009. Inhibitory effects of ketamine on lipopolysaccharide-induced microglial activation. Mediators Inflamm. 2009, 705379 ( 10.1155/2009/705379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norton WT, Poduslo SE. 1973. Myelination in rat brain: method of myelin isolation. J. Neurochem. 21, 749–757. [DOI] [PubMed] [Google Scholar]
- 32.Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, Bar-Or A, Antel JP. 2012. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia 60, 717–727. ( 10.1002/glia.22298) [DOI] [PubMed] [Google Scholar]
- 33.Pêgo AP, Vleggeert-Lankamp CLAM, Deenen M, Lakke EAJF, Grijpma DW, Poot AA, Marani E, Feijen J. 2003. Adhesion and growth of human Schwann cells on trimethylene carbonate (co)polymers. J. Biomed. Mater. Res. A 67, 876–885. ( 10.1002/jbm.a.10074) [DOI] [PubMed] [Google Scholar]
- 34.Streit WJ, Walter SA, Pennell NA. 1999. Reactive microgliosis. Prog. Neurobiol. 57, 563–581. ( 10.1016/s0301-0082(98)00069-0) [DOI] [PubMed] [Google Scholar]
- 35.Shechter R, Schwartz M. 2013. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer if ‘but how’. J. Pathol. 229, 332–346. ( 10.1002/path.4106) [DOI] [PubMed] [Google Scholar]
- 36.Sofroniew MV, Vinters HV. 2010. Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35. ( 10.1007/s00401-009-0619-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang D, Hu X, Qian L, O'Callaghan JP, Hong JS. 2010. Astrogliosis in CNS pathologies: is there a role for microglia? Mol. Neurobiol. 41, 232–241. ( 10.1007/s12035-010-8098-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duarri A, et al. 2011. Knockdown of MLC1 in primary astrocytes causes cell vacuolation: a MLC disease cell model. Neurobiol. Dis. 43, 228–238. ( 10.1016/j.nbd.2011.03.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liesi P, Kauppila T. 2002. Induction of type IV collagen and other basement-membrane-associated proteins after spinal cord injury of the adult rat may participate in formation of the glial scar. Exp. Neurol. 173, 31–45. ( 10.1006/exnr.2001.7800) [DOI] [PubMed] [Google Scholar]
- 40.Boche D, Perry VH, Nicoll JAR. 2013. Review: activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 39, 3–18. ( 10.1111/nan.12011) [DOI] [PubMed] [Google Scholar]
- 41.Lively S, Schlichter LC. 2013. The microglial activation state regulates migration and roles of matrix-dissolving enzymes for invasion. J. Neuroinflam. 10, 75 ( 10.1186/1742-2094-10-75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szabo M, Gulya K. 2013. Development of the microglial phenotype in culture. Neuroscience 241, 280–295. ( 10.1016/j.neuroscience.2013.03.033) [DOI] [PubMed] [Google Scholar]
- 43.Torres-Platas SG, Comeau S, Rachalski A, Bo GD, Cruceanu C, Turecki G, Giros B, Mechawar N. 2014. Morphometric characterization of microglial phenotypes in human cerebral cortex. J. Neuroinflamm. 11, 12 ( 10.1186/1742-2094-11-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartneck M, Heffels KH, Pan Y, Bovi M, Zwadlo-Klarwasser G, Groll J. 2012. Inducing healing-like human primary macrophage phenotypes by 3D hydrogel coated nanofibres. Biomaterials 33, 4136–4146. ( 10.1016/j.biomaterials.2012.02.050) [DOI] [PubMed] [Google Scholar]
- 45.Saino E, Focarete ML, Gualandi C, Emanuele E, Cornaglia AI, Imbriani M, Visai L. 2011. Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromolecules 12, 1900–1911. ( 10.1021/bm200248h) [DOI] [PubMed] [Google Scholar]
- 46.Chen S, Jones JA, Xu Y, Low HY, Anderson JM, Leong KW. 2010. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials 31, 3479–3491. ( 10.1002/jbm.a.32609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. 2013. Modulation of macrophage phenotype by cell shape. Proc. Natl Acad. Sci. USA 110, 17 253–17 258. ( 10.1073/pnas.1308887110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lambert C, Ase AR, Séguéla P, Antel JP. 2010. Distinct migratory and cytokine responses of human microglia and macrophages to ATP. Brain Behav. Immun. 24, 1241–1248. ( 10.1016/j.bbi.2010.02.010) [DOI] [PubMed] [Google Scholar]
- 49.Pan XD, Zhu YG, Lin N, Zhang J, Ye QY, Huang HP, Chen XC. 2011. Microglial phagocytosis induced by fibrillar β-amyloid is attenuated by oligomeric β-amyloid: implications for Alzheimer's disease. Mol. Neurodegener. 6, 45 ( 10.1186/1750-1326-6-45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hart AD, Wyttenbach A, Hugh Perry V, Teeling JL. 2012. Age related changes in microglial phenotype vary between CNS regions: grey versus white matter differences. Brain Behav. Immun. 26, 754–765. ( 10.1016/j.bbi.2011.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tambuyzer BR, Ponsaerts P, Nouwen EJ. 2009. Microglia: gatekeepers of central nervous system immunology. J. Leukoc. Biol. 85, 352–370. ( 10.1189/jlb.0608385) [DOI] [PubMed] [Google Scholar]
- 52.Hornik TC, Neniskyte U, Brown GC. 2014. Inflammation induces multinucleation of microglia via PKC inhibition of cytokinesis, generating highly phagocytic multinucleated giant cells. J. Neurochem. 128, 650–661. ( 10.1111/jnc.12477) [DOI] [PubMed] [Google Scholar]
- 53.Mosley K, Cuzner ML. 1996. Receptor-mediated phagocytosis of myelin by macrophages and microglia: effect of opsonization and receptor blocking agents. Neurochem. Res. 21, 481–487. ( 10.1007/bf02527713) [DOI] [PubMed] [Google Scholar]
- 54.Beyer M, Gimsa U, Eyüpoglu IY, Hailer NP, Nitsch R. 2000. Phagocytosis of neuronal or glial debris by microglial cells: upregulation of MHC class II expression and multinuclear giant cell formation in vitro. Glia 31, 262–266. () [DOI] [PubMed] [Google Scholar]
- 55.Almeida CR, Serra T, Oliveira MI, Planell JA, Barbosa MA, Navarro M. 2014. Impact of 3-D printed PLA- and chitosan-based scaffolds on human monocyte/macrophage responses: unraveling the effect of 3-D structures on inflammation. Acta Biomater. 10, 613–622. ( 10.1016/j.actbio.2013.10.035) [DOI] [PubMed] [Google Scholar]
- 56.Gao Z, Zhu Q, Zhang Y, Zhao Y, Cai L, Shields CB, Cai J. 2013. Reciprocal modulation between microglia and astrocyte in reactive gliosis following the CNS injury. Mol. Neurobiol. 48, 690–701. ( 10.1007/s12035-013-8460-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buffo A, Rolando C, Ceruti S. 2010. Astrocytes in the damaged brain: molecular and cellular insights into their reactive response and healing potential. Biochem. Pharmacol. 79, 77–89. ( 10.1016/j.bcp.2009.09.014) [DOI] [PubMed] [Google Scholar]
- 58.Röhl C, Lucius R, Sievers J. 2007. The effect of activated microglia on astrogliosis parameters in astrocyte cultures. Brain Res. 1129, 43–52. ( 10.1016/j.brainres.2006.10.057) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.