Abstract
The transcription factor LMO2 is involved in vascular and hematopoietic development and hematolymphoid neoplasia. We have demonstrated that LMO2 is expressed nearly ubiquitously in native and neoplastic vasculature, including lymphatics. LMO2 reactivity is otherwise virtually absent in nonhematolymphoid tissues except in breast myoepithelium, prostatic basal cells, and secretory phase endometrial glands. Vasculature is LMO2− in adult and fetal heart, brain of older adults, hepatic sinusoids, and hepatocellular carcinoma. LMO2 is uniformly expressed in benign vascular and lymphatic neoplasms and in most malignant vascular neoplasms with the exception of epithelioid vascular neoplasms of pleura and bone. Among nonvascular neoplasms, LMO2 reactivity is present in giant cell tumor of tendon sheath, juvenile xanthogranuloma, a subset of gastrointestinal stromal tumors, small round blue cell tumors, and myoepithelial-derived neoplasms. The restricted expression pattern, nuclear localization, and crisp staining of LMO2 in paraffin blocks make it an attractive candidate for the diagnostic immunohistochemistry laboratory.
Keywords: LMO2, Endothelium, Angiosarcoma, Myoepithelial, Giant cell tumor of tendon sheath, Gastrointestinal stromal tumor
LMO2 is a member of a transcription factor family of proteins characterized by their cysteine-rich, zinc-binding LIM domains.1 Its expression is required early in hematopoiesis,2 and its deregulation leads to T-cell leukemias resulting from chromosomal translocations and insertional mutations.3,4 LMO2 expression is a strong predictor of superior outcome in patients with diffuse large B-cell lymphoma.5,6 In addition to its pivotal roles in hematopoiesis and hematopoietic malignancy, LMO2 is required for angiogenesis early in development: the primitive vascular network formed by vasculogenesis does not undergo maturation into functional vascular structures in the absence of LMO2.7 We recently developed a monoclonal anti-LMO2 antibody suitable for paraffin immunohistochemical studies8 and demonstrated that the LMO2 protein is expressed in normal germinal center B cells and germinal center–derived B-cell lymphomas. We also observed expression of LMO2 in vascular endothelium, but not in other nonhematolymphoid tissues.8
In the present study, we set out to assess the usefulness of LMO2 protein as a marker of vascular differentiation and to assess the expression of LMO2 in the vasculature of a variety of native and neoplastic tissues. We have confirmed expression of LMO2 in selected native tissues by immunoblotting and have extensively surveyed LMO2 protein expression by immunohistochemical studies in 119 native tissue specimens representing 21 sites, 28 benign vascular neoplasms representing 9 distinct entities, 47 malignant vascular neoplasms representing 7 distinct entities, and 617 nonvascular neoplasms representing 62 distinct entities.
Materials and Methods
Tissue Samples
Formalin-fixed, paraffin-embedded normal and neoplastic tissues were obtained from the archives of the Departments of Pathology, Stanford University Medical Center, Stanford, CA. Tissue microarrays (TMAs) representing 64 native tissues (adrenal gland, 4; breast, 1; gastrointestinal, 11; heart, 1; kidney, 3; liver, 5; lung, 3; lymph node, 7; ovary, 2; pancreas, 2; parathyroid, 3; placenta, 3; skeletal muscle, 3; spleen, 3; thymus, 3; tonsil, 7; and uterus, 3), 21 vascular neoplasms, and 561 nonvascular neoplasms (bladder, 8; bone, 25; breast, 15; cervix, 4; colon, 3; endometrium, 13; kidney, 27; liver, 9; lung, 8; ovary, 10; pancreas, 6; pleura, 4; prostate, 2 Gleason score 6 and 1 Gleason score 7; salivary gland, 3; small round blue cell tumor, 14; smooth muscle, 78; soft tissue, 318; and skin, 13) were previously constructed as described9 using a tissue arrayer10 (Beecher Instruments, Silver Spring, MD).
An additional 167 whole tissue sections, including 55 native tissues (brain/spinal cord, 14; breast, 4; cervix, 1; colon, 1; coronary artery, 1; endometrium, 2; heart, 5; kidney, 2; liver, 3; lung, 1; placenta, 1; prostate, 11; salivary gland, 3; skeletal muscle, 2; skin, 2; thymus, 1; and tonsil, 1) and 112 neoplasms (benign vascular, 21; malignant vascular, 35; brain, 25; breast, 3; colon, 1; epithelial/myoepithelial neoplasms, 5; lung, 1; kidney, 2; liver, 1; pleura, 1; prostate, 4 Gleason score 6, 1 Gleason score 8, 5 Gleason score 9, and 1 Gleason score 10; skin, 2; and soft tissue, 4) were used to confirm and extend the findings based on the TMA material. Seven fresh tissue samples were obtained from surgical specimens (lung, thymus, tonsil, liver) or autopsy specimens (3 frontal cortex specimens, from a neonate, a 63-year-old, and a 70-year-old) and were immediately frozen at −70°C for immunoblot analysis. Institutional review board approval was obtained for these studies.
Immunohistochemical Analysis
Immunohistochemical analysis was performed on 4-µm sections of whole tissues or TMAs that were placed on glass slides, baked for 1 hour at 60°C, deparaffinized in xylene, and hydrated in a series of graded alcohols. A monoclonal mouse antihuman LMO2 antibody8 at a dilution of 1:150 was used for these studies. Immunohistochemical studies for LMO2 were performed using mild retrieval on a Ventana BenchMark automated stainer (Ventana, Tucson, AZ). Additional biotin blockade was not undertaken. The CD34 antibody (clone MY10, Becton Dickinson Biosciences, San Jose, CA) was used at a dilution of 1:60 under mild retrieval conditions on a Ventana BenchMark automated stainer. The CD31 antibody (clone JC/70A, DAKO, Carpinteria, CA) was used at a dilution of 1:150 under standard retrieval conditions. The D2-40 antibody was used at a dilution of 1:40 without retrieval on the DAKO Autostainer (DAKO).
Immunoblot Analysis
Whole tissue extracts of fresh frozen specimens of brain, lung, liver, thymus, and tonsil were minced and filtered through a cell strainer (BD Falcon, San Jose, CA). Immunoblot analysis was then performed as previously described.8
Image Acquisition
Microscopic images were acquired using a Nikon Eclipse E400 microscope (Nikon, Tokyo, Japan) with a 40×/0.75 NA Plan Fluor objective lens. Digitized images were processed using Adobe Photoshop 7 software (Adobe Systems, San Jose, CA).
Results
LMO2 Is Widely Expressed in the Vasculature of Native Tissues, Including Lymphatic Vasculature
We surveyed the expression of LMO2 in the vasculature of a variety of native tissues summarized in Table 1, with selected examples depicted in Image 1. Immunohistochemical staining for LMO2 was distinct and was limited to the nucleus in vasculature. LMO2 immunoreactivity was present in microvasculature, venous and arterial vasculature, and lymphatic endothelium. Expression of LMO2 was documented in the vasculature of brain, breast, endocrine organs (including adrenal, pancreas, and parathyroid), gastrointestinal tract (including the appendix, colon, small bowel, and stomach), hematolymphoid tissues (including lymph node, tonsil, spleen, and thymus), kidney (glomerular and intertubular endothelium), liver (portal tract vasculature), lung, ovary, placenta, prostate, salivary gland, skeletal muscle, and uterus (endometrium and myometrium).
Table 1.
LMO2 Expression in Native Tissues
| Tissue Type | Vessels | Tissues |
|---|---|---|
| Adrenal | 4/4 | 0/4 |
| Brain | ||
| Adult >60 y | 0/3 | 0/3 |
| Adult <45 y | 4/4 | 0/4 |
| Child | 3/3 | 0/3 |
| Fetal | 2/2 | 0/2 |
| Breast* | 5/5 | 5/5 |
| Endometrium | ||
| Proliferative | 5/5 | 0/5 |
| Secretory† | 2/2 | 2/2 |
| Gastrointestinal tract | 12/12 | 0/12 |
| Kidney | 5/5 | 0/5 |
| Heart | ||
| Adult | 0/4 | 0/4 |
| Fetal | 0/2 | 0/2 |
| Liver | ||
| Portal tract | 3/3 | 0/3 |
| Sinusoids | 0/8 | 0/8 |
| Lung | 4/4 | 0/4 |
| Lymph node | 7/7 | 7/7 |
| Ovary | 2/2 | 0/2 |
| Pancreas | 2/2 | 0/2 |
| Parathyroid | 3/3 | 0/3 |
| Placenta | 4/4 | 0/4 |
| Prostate‡ | 11/11 | 0/11 |
| Salivary gland | 3/3 | 0/3 |
| Skeletal muscle | 5/5 | 0/5 |
| Skin | 2/2 | 0/2 |
| Spleen | 3/3 | 3/3 |
| Tonsil | 8/8 | 8/8 |
| Thymus | 4/4 | 4/4 |
Breast myoepithelium positive.
Secretory endometrial glands positive.
Basal layer focally positive.
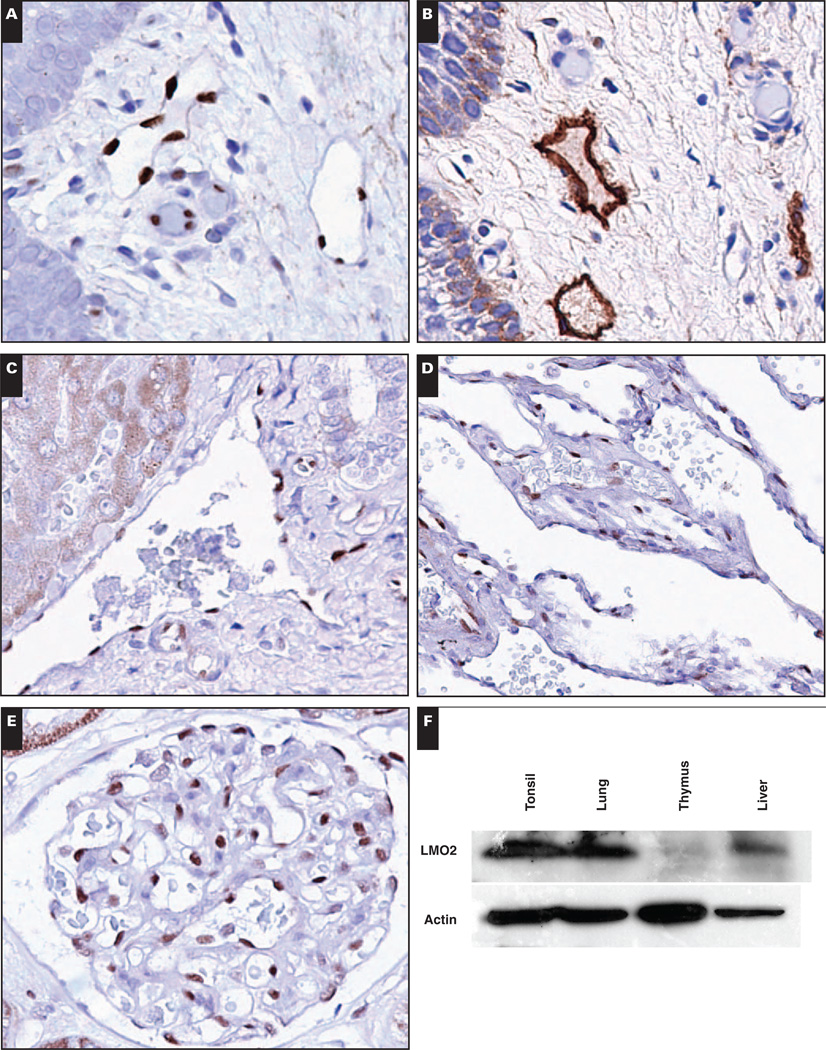
Image 1.
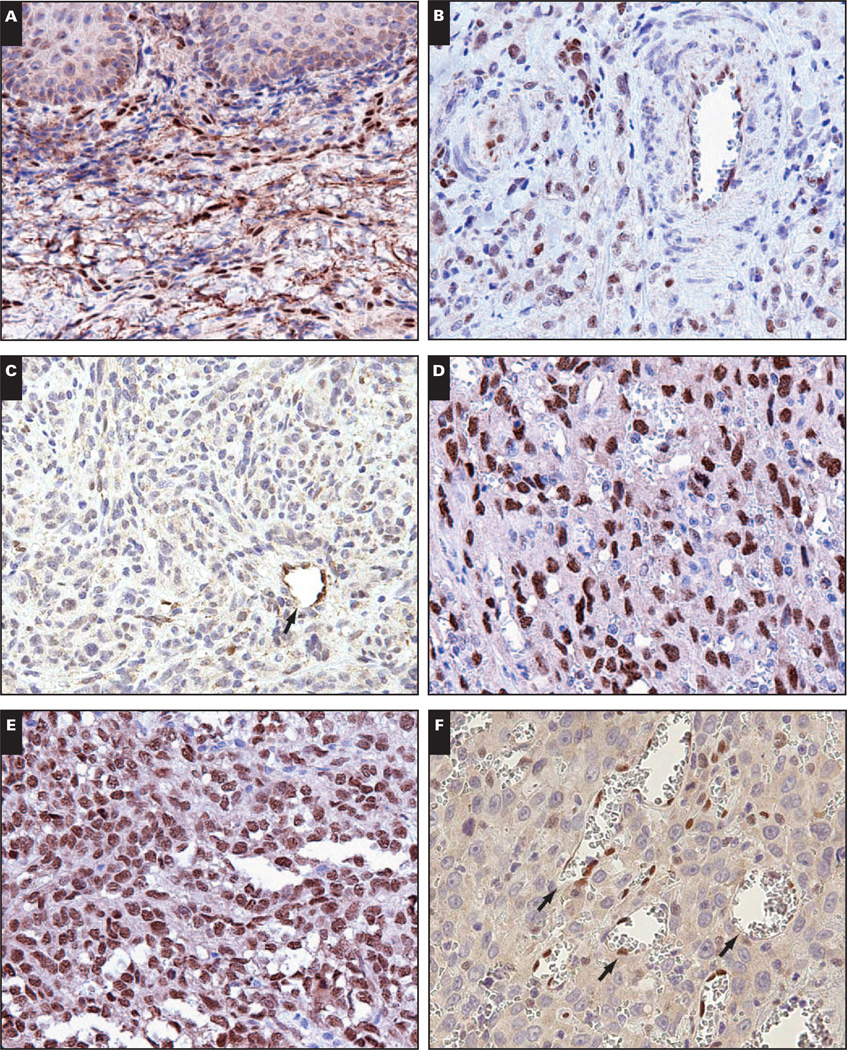
Native tissue vasculature, including lymphatic vasculature, is generally LMO2+. A, Tonsil shows LMO2+ blood vessels and lymphatic vessels (×400). B, Tonsil stained with lymphatic marker D2-40 to highlight lymphatic vessels (×400). C, Liver shows LMO2+ portal arteries and veins and LMO2− sinusoids (×400). D and E, Lung (D, ×400) and kidney (E, ×400) have LMO2+ vasculature. F, Expression of LMO2 in native tissues by immunoblotting. Actin serves as a loading control.
LMO2 expression has not previously been described in mature human nonneoplastic tissue vasculature, and we therefore confirmed its expression in native tissues by immunoblotting. Tonsil was used as a positive control sample; this represents LMO2 expression in germinal center lymphocytes and in vasculature as demonstrated by immunohistochemical studies. Liver, lung, and thymus, however, showed LMO2 expression that was restricted to vascular endothelium by immunohistochemical studies; immunoblotting confirmed the presence of LMO2 protein, more so in highly vascular tissues (lung and liver) than in thymus (Image 1F).
Exceptions to the Expression of LMO2 in Native Vasculature
The majority of native tissues showed ubiquitous expression of LMO2 in vasculature, with 3 exceptions: heart, brain in older adults, and hepatic sinusoidal endothelium (Table 1). Portal tract veins and arteries were LMO2+, but the hepatic sinusoidal endothelium was negative for LMO2 (Image 1C). Rare LMO2+ mononuclear cells within sinusoids likely represent monocyte/macrophage lineage cells (Kupffer cells).
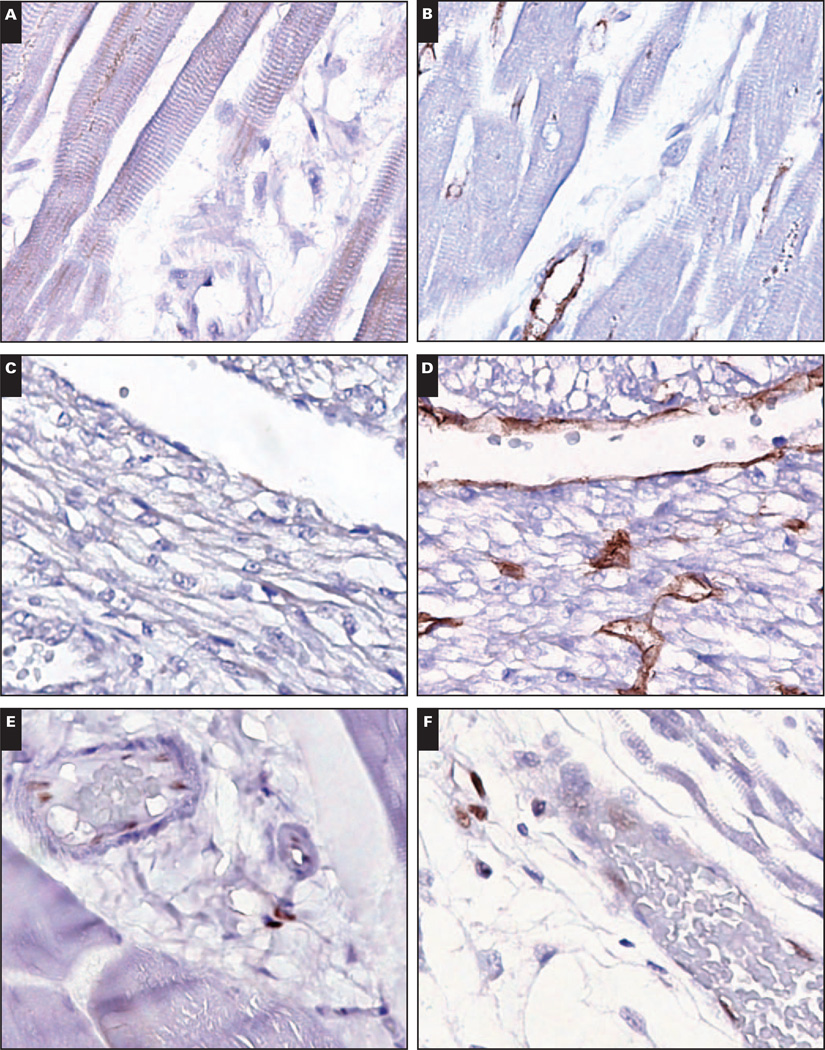
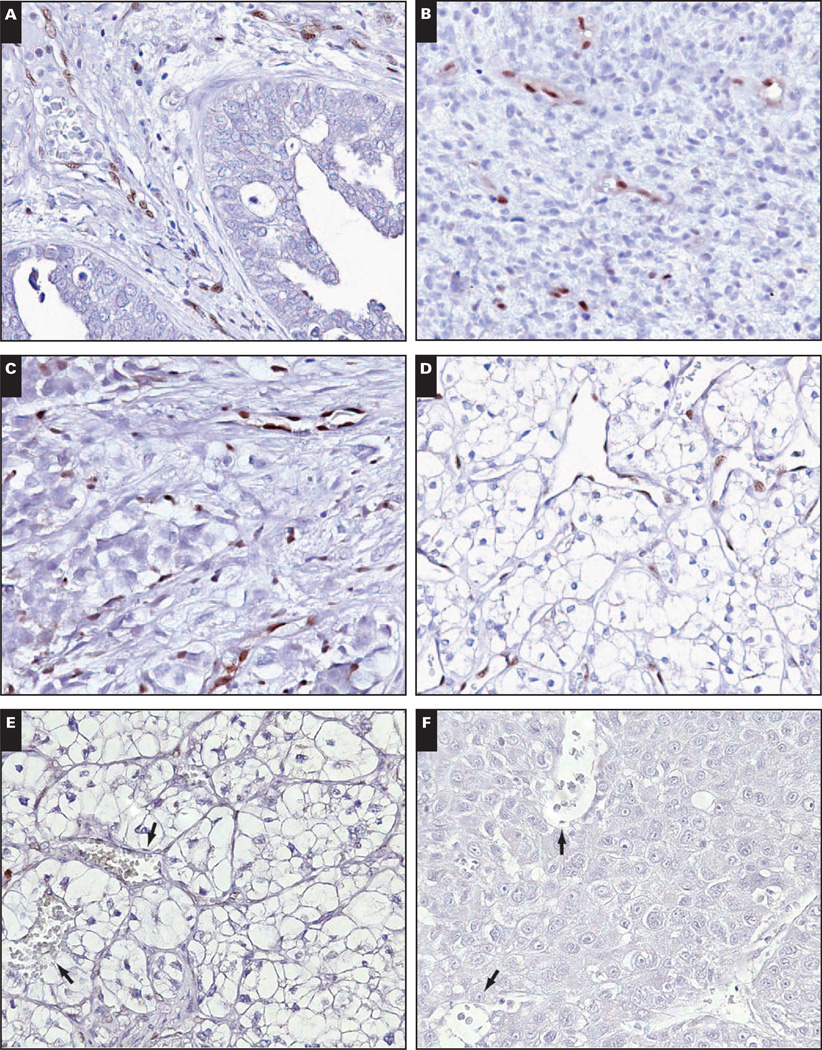
Skeletal muscle vasculature was LMO2+. In contrast, myocardial vasculature and endocardium lacked LMO2 in fetal and adult heart tissue obtained at autopsy Image 2. CD34 immunostaining was performed to confirm tissue immunogenicity and was positive in myocardial vasculature and endocardium in all cases. In addition, 1 myocardial specimen was embedded in a single paraffin block with concurrently obtained samples of skeletal muscle, thyroid, and thymus tissue, which all showed LMO2+ vasculature (Images 2E and 2F, heart and skeletal muscle from the same paraffin block).
Image 2.
Myocardial vasculature is LMO2−. A, Adult myocardial vasculature is LMO2− (×400). B, The rich microvasculature is evident on CD31 immunostain (×400). C, Similarly, fetal myocardial vasculature is LMO2− (×400). D, CD31 highlights the vasculature (×400). E and F, By contrast, adult skeletal muscle vasculature (E, ×400) and fetal skeletal muscle vasculature (F, ×400) are LMO2+.
Brain tissues from fetuses, children (ages 2–17 years), and adults (ages 21–43 years) showed unequivocal LMO2 expression in brain vasculature, in white and gray matter. In contrast, brain and spinal cord tissues from older adults (ages 64–76 years) lacked demonstrable LMO2 in vascular endothelium by immunostaining. Positive internal controls were present in the form of LMO2+ subarachnoid vessels and LMO2+ vessels within nerve roots Image 3A, Image 3B, Image 3C, Image 3D, and Image 3E. CD34 immunostaining was performed to confirm tissue immunogenicity and was positive in brain and spinal cord vasculature in all LMO2− cases (data not shown).
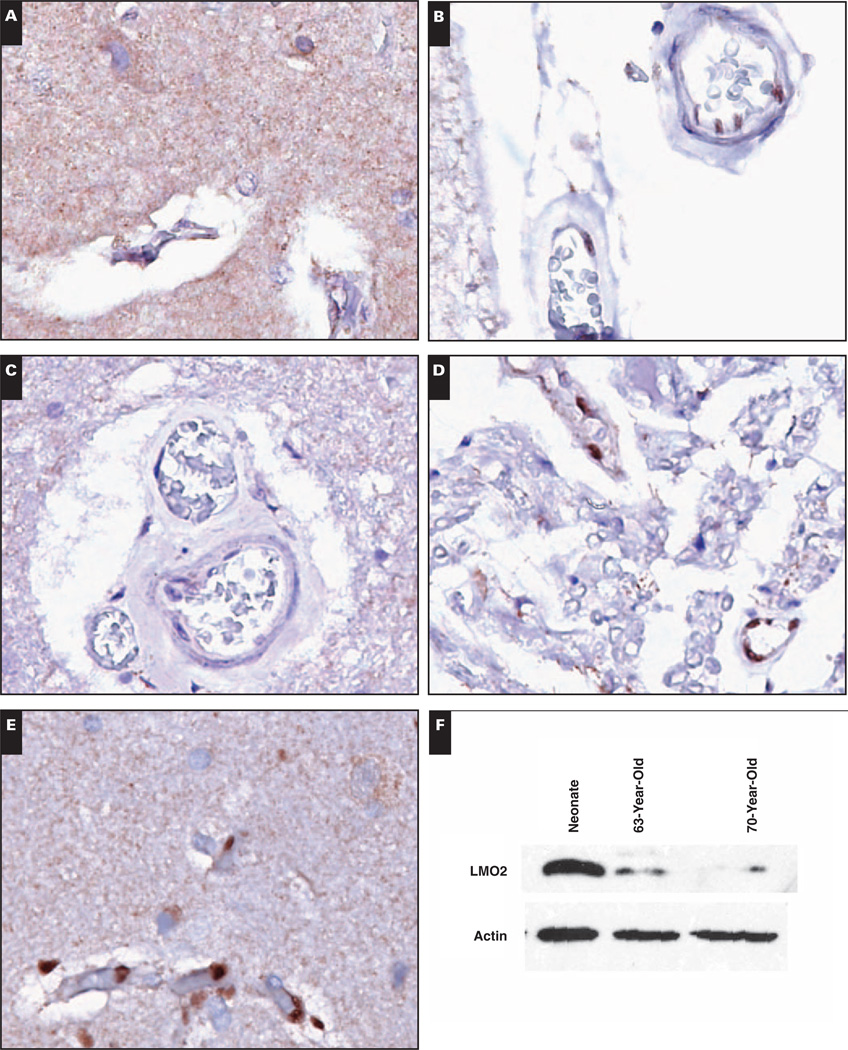
Image 3.
Central nervous system vasculature loses LMO2 reactivity in older adults. A and B, The cortex of a 73-year-old adult shows LMO2− vasculature (A, ×400) with overlying LMO2+ subarachnoid vessels (B, ×400). C and D, The spinal cord from the 73-year-old adult shows LMO2− vessels (C, ×400) with adjacent nerve roots showing LMO2+ vasculature (D, ×400). E, The cortex of a 39-year-old adult shows LMO2+ vasculature (×400). F, Expression of LMO2 in brain tissue by immunoblotting. Actin serves as a loading control.
To confirm age-related loss of reactivity for LMO2, we performed immunoblotting for LMO2 on fresh brain tissue obtained at autopsy. A specimen from a neonate was used as a positive control sample and showed strong expression of LMO2, whereas expression of LMO2 was much lower in specimens obtained from older adults Image 3F.
LMO2 Expression in Benign Vascular Neoplasms
Uniform nuclear expression of LMO2 was noted in a variety of benign vascular neoplasms of blood and lymphatic vascular derivation Table 2 and Image 4, including capillary hemangioma, infantile hemangioma, infantile hemangioendothelioma of liver, littoral cell angioma, and lym-phangioma. The lymphatic derivation of the lymphangiomas tested was confirmed by immunostaining with D2-40 (data not shown).
Table 2.
LMO2 Expression in Vascular Neoplasms
| LMO2 | CD31 | CD34 | |
|---|---|---|---|
| Benign | |||
| Angiomatosis | 1/1 | 1/1 | 1/1 |
| Hemangioma | 14/14 | 8/8 | 6/6 |
| Capillary | 5/5 | 4/4 | 3/3 |
| Infantile | 5/5 | 1/1 | 1/1 |
| Epithelioid | 1/1 | 1/1 | 1/1 |
| Histiocytoid | 1/1 | — | — |
| Intramuscular | 1/1 | 1/1 | 1/1 |
| Splenic | 1/1 | 1/1 | — |
| Infantile hemangioendothelioma | 3/3 | — | — |
| Littoral cell angioma | 2/2 | 2/2 | — |
| Lymphangioma | 8/8 | 1/2 | — |
| Malignant | |||
| Angiosarcoma | 25/28 | 17/17 | 8/15 |
| Epithelioid | 4/6 | 6/6 | 1/6 |
| of Pleura | 0/2 | 2/2 | 0/2 |
| Not otherwise specified | 21/22 | 11/11 | 7/9 |
| Hemangioendothelioma | 7/12 | 11/11 | 6/9 |
| Epithelioid | 6/10 | 10/10 | 5/8 |
| of Bone | 1/4 | 4/4 | 3/4 |
| of Pleura | 0/1 | 1/1 | 0/1 |
| of Other sites | 5/5 | 5/5 | 2/3 |
| Spindled | 1/2 | 1/1 | 1/1 |
| Kaposi sarcoma | 7/7 | 4/4 | 4/4 |
Image 4.
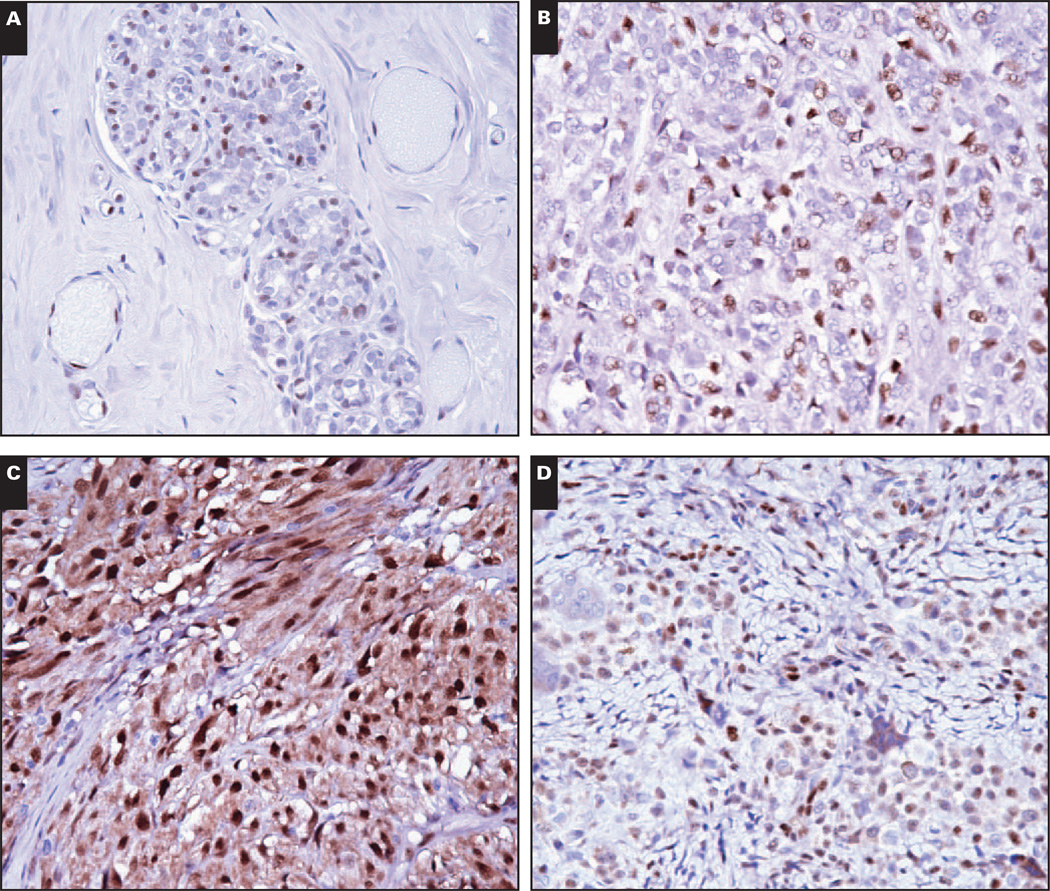
Benign vascular neoplasms, including lymphovascular neoplasms, are uniformly LMO2+. A, Capillary hemangioma (×200). B, Epithelioid hemangioma (×200). C, Littoral cell angioma (×200). B, Lymphangioma (×200).
LMO2 Expression in Malignant Vascular Neoplasms
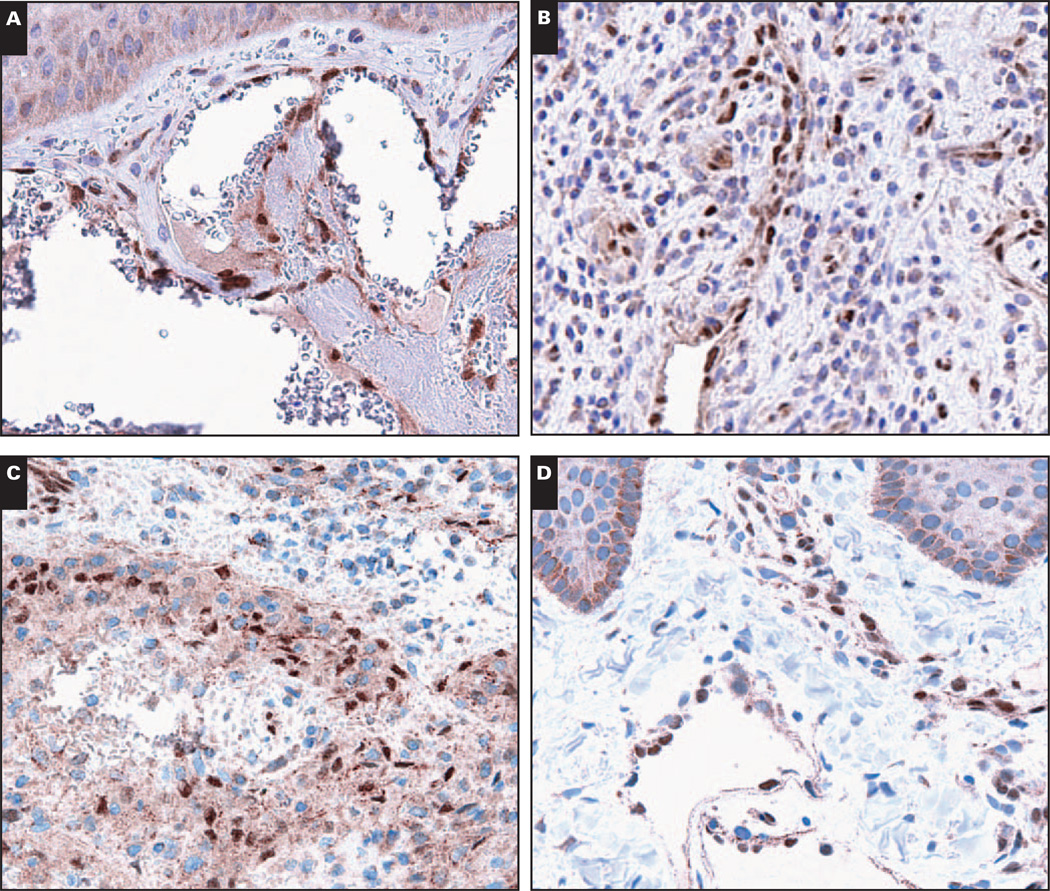
We next surveyed LMO2 expression in malignant vascular neoplasms originating from a wide variety of anatomic sites, including brain, bone, breast, colon, liver, skin/subcutaneous tissue, and pleura (Table 2) Image 5. All Kaposi sarcomas, 7/12 hemangioendotheliomas, and 25/28 angiosarcomas showed staining for LMO2. Among epithelioid vascular malignancies (epithelioid hemangioendotheliomas and epithelioid angiosarcomas), a subset (6 of 16) lacked nuclear LMO2. All epithelioid vascular malignancies were immunoreactive for CD31, confirming their vascular derivation (Table 2). Among vascular malignancies, the lack of LMO2 was limited to 2 entities: epithelioid hemangioendotheliomas of bone (3 of 4 negative for LMO2, Image 5C) and epithelioid vascular malignancies of pleura (1 epithelioid hemangioendothelioma and 2 epithelioid angiosarcomas, Image 5F). In contrast, all 9 epithelioid vascular malignancies originating at other sites were LMO2+ (Images 5B and 5E). These included 5 epithelioid hemangioendotheliomas originating in brain, liver, mediastinum, and subcutaneous tissue and 4 epithelioid angiosarcomas originating in the neck, mediastinum, pericardium, and soft tissues. The lack of LMO2 expression in epithelioid vascular malignancies originating in bone and pleura compared with the uniform expression of LMO2 in epithelioid vascular malignancies originating elsewhere was statistically significant (P < .002; and χ2= 12.8 for the 3-way comparison).
Image 5.
Malignant vascular neoplasms are LMO2+ with the exception of epithelioid vascular malignancies of bone and pleura. A, B, and C, Kaposi sarcoma (A, ×200) and epithelioid hemangioendothelioma associated with a muscular arteriole (B, ×200) are LMO2+, whereas an epithelioid hemangioendothelioma of bone (C, ×200) is LMO2− (arrow, internal positive control tumor vasculature). D, E, and F, An angiosarcoma of the colon (D, ×200) and a pericardial epithelioid angiosarcoma (E, ×200) are LMO2+, whereas a pleural epithelioid angiosarcoma (F, ×200) is LMO2− (arrows, internal positive control tumor vasculature).
LMO2 Expression in the Vasculature of Neoplasms
We surveyed LMO2 expression in the vasculature of a wide range of neoplasms Table 3 and Image 6, including a variety of carcinomas and sarcomas, other soft tissue neoplasms, mesothelioma, malignant melanoma, and primary neoplasms of bone and brain. LMO2 was expressed in the vasculature of all neoplasms assessed with 2 exceptions: hepatocellular carcinoma (9/10) lacked staining for LMO2 in the vasculature, as did a subset of clear cell renal cell carcinoma (8/23). There was no relationship between vascular LMO2 expression and Fuhrman grade (data not shown).
Table 3.
LMO2 Expression in Nonvascular Neoplasms
| Neoplasm | Tumor | Vessel |
|---|---|---|
| Carcinoma | ||
| Breast (ductal) | 0/5 | 5/5 |
| Cervical | 0/4 | 4/4 |
| Colon | 0/4 | 4/4 |
| Kidney (clear cell) | 0/23 | 15/23 |
| Liver (hepatocellular) | 0/10 | 1/10 |
| Lung | 0/9 | 9/9 |
| Pancreas (ductal) | 0/6 | 6/6 |
| Prostate | 0/14 | 14/14 |
| Transitional cell | 0/8 | 7/8 |
| Uterus, endometrial | 0/2 | 2/2 |
| Uterus, carcinosarcoma | 1/7 | 7/7 |
| Bone | ||
| Giant cell tumor of bone | 2/7 | 7/7 |
| Nonossifying fibroma | 0/6 | 6/6 |
| Osteosarcoma | 0/12 | 10/11 |
| Brain | ||
| Capillary hemangioblastoma | 0/4 | 4/4 |
| Glioblastoma multiforme | 0/5 | 5/5 |
| Medulloblastoma | 0/3 | 3/3 |
| Oligodendroglioma | 0/4 | 4/4 |
| Pilocytic astrocytoma | 0/4 | 4/4 |
| Pituitary adenoma | 0/5 | 5/5 |
| Epithelial-myoepithelial | ||
| Adenoid cystic carcinoma | 0/3 | 2/2 |
| Epithelial-myoepithelial carcinoma | 1/1 | 1/1 |
| Myoepithelioma | 0/2 | 2/2 |
| Pleomorphic adenoma | 2/2 | 2/2 |
| Small round blue cell | ||
| Neuroblastoma | 0/3 | 3/3 |
| Ewing sarcoma | 3/6 | 3/4 |
| Desmoplastic small round cell tumor | 3/5 | 4/5 |
| Soft tissue/miscellaneous | ||
| Angiomyolipoma | 0/3 | 3/3 |
| Dermatofibrosarcoma protuberans | 0/8 | 8/8 |
| Desmoid-type fibromatosis | 0/26 | 26/26 |
| Digital fibromatosis | 0/4 | 4/4 |
| Endometrial stromal sarcoma | 0/4 | 4/4 |
| Epithelioid sarcoma | 2/7 | 7/7 |
| Extraskeletal myxoid chondrosarcoma | 0/8 | 6/6 |
| Fibroadenoma | 0/11 | 11/11 |
| Fibroma of tendon sheath | 0/8 | 8/8 |
| Fibrous dysplasia | 0/9 | 8/9 |
| Gastrointestinal stromal tumor* | 33/57 | 41/42 |
| Giant cell tumor of tendon sheath | 26/30 | 30/30 |
| Glomus tumor | 0/4 | 4/4 |
| Hemangiopericytoma | 0/2 | 2/2 |
| Inflammatory myofibroblastic tumor | 0/4 | 4/4 |
| Juvenile xanthogranuloma | 2/2 | 2/2 |
| Leiomyoma | 0/19 | 18/19 |
| Leiomyosarcoma | 0/59 | 54/54 |
| Liposarcoma | 0/32 | 25/25 |
| Low-grade fibromyxoid sarcoma | 0/3 | 3/3 |
| Malignant fibrous histiocytoma | 1/65 | 64/65 |
| Malignant melanoma | 0/4 | 4/4 |
| Malignant mesothelioma | 0/5 | 5/5 |
| Malignant peripheral nerve sheath tumor | 1/7 | 7/7 |
| Myxofibrosarcoma | 0/7 | 4/4 |
| Myxoma | 0/6 | 5/5 |
| Neurofibroma | 0/12 | 12/12 |
| Nodular fasciitis | 0/7 | 7/7 |
| Ovarian fibroma | 0/10 | 1010 |
| Phyllodes tumor | 0/2 | 2/2 |
| Rhabdomyosarcoma | 1/10 | 10/10 |
| Schwannoma | 0/18 | 18/18 |
| Solitary fibrous tumor | 0/19 | 19/19 |
| Synovial sarcoma | 3/20 | 19/20 |
| Wilms tumor | 0/2 | 0/2 |
33/57 with nuclear staining; 43/57 with nuclear or cytoplasmic staining.
Image 6.
Nonvascular malignancies have LMO2+ vasculature, except hepatocellular carcinoma and a subset of clear cell renal cell carcinomas. A, B, and C, Lung adenocarcinoma (A, ×400), glioblastoma (B, ×400), and mesothelioma (C, ×400) have LMO2+ vasculature. D and E, Clear cell renal cell carcinomas may have LMO2+ (D, ×400) or LMO2− (E, ×400) vasculature. F, Hepatocellular carcinoma has LMO2− vasculature (arrows, LMO2− vessels; ×400).
Extravascular LMO2 Expression in Native Tissues
LMO2 expression in native tissues (Table 1) was restricted to vasculature and hematolymphoid cells with 3 specific exceptions: nuclear LMO2 expression was noted in breast myoepithelial cells Image 7A, prostate gland basal cells, and endometrial glands in secretory phase but not proliferative phase endometrium. While breast myoepithelial cells were consistently LMO2+, prostate gland basal cell LMO2 reactivity was patchy. In contrast with breast myoepithelium, salivary gland myoepithelium and myoepithelial cells associated with skin adnexal structures were LMO2−.
Image 7.
LMO2 reactivity outside the vasculature in myoepithelium and derived neoplasms, gastrointestinal stromal tumor, and giant cell tumor of tendon sheath. A, Benign breast lobule with LMO2+ myoepithelial cells (×400). B, Epithelial-myoepithelial carcinoma demonstrating LMO2 reactivity in the more spindled myoepithelial component (×400). C, Gastrointestinal stromal tumor showing nuclear and cytoplasmic staining (×400). D, Giant cell tumor of tendon sheath (×400).
LMO2 Expression in Nonvascular Neoplasms
We screened a very broad array of epithelial and nonepithelial neoplasms for LMO2 expression and found nuclear LMO2 expression to be rare in most entities (Table 3) Image 7B, Image 7C, and Image 7D. Notable exceptions included giant cell tumor of tendon sheath, juvenile xanthogranuloma, more than half of the cases of gastrointestinal stromal tumor (GIST), a subset of epithelial-myoepithelial neoplasms (pleomorphic adenoma and epithelial-myoepithelial carcinoma), and a subset of small round blue cell tumors (Ewing sarcoma and desmoplastic small round blue cell tumor). Giant cell tumor of tendon sheath showed staining in mononuclear cells but not in multinucleated giant cells. Staining was uniformly present in diffuse-type giant cell tumor or pigmented villonodular synovitis (8/8) and in most cases of localized type tenosynovial giant cell tumor (18/22). GISTs exhibited a distinctive LMO2 staining pattern: in addition to the usual nuclear staining pattern seen in vasculature and in most neoplasms, more than half of the GIST cases showed strong diffuse cytoplasmic staining (often, but not always, in combination with nuclear staining).
Neoplasms with occasional LMO2+ examples included epithelioid sarcoma (2/7, weak staining), giant cell tumor of bone (2/7), and synovial sarcoma (3/20). Rare cases of some poorly differentiated or high-grade neoplasms were LMO2-reactive: 1 of 7 carcinosarcomas, 1 of 65 malignant fibrous histiocytomas, 1 of 7 malignant peripheral nerve sheath tumors, and 1 of 10 rhabdomyosarcomas. LMO2+ infiltrating mononuclear cells were frequently noted in benign peripheral nerve sheath tumors, including neurofibromas and schwannomas, but the neoplastic spindled cells themselves were LMO2−. Infiltrating LMO2+ mononuclear cells were not noted in malignant peripheral nerve sheath tumors or other entities. Finally, although LMO2 staining was very crisp and free of background, we noted that myxoid neoplasms including myxoma, extraskeletal myxoid chondrosarcoma, and chondromyxoid fibroma showed spurious staining in the form of round blobs of cytoplasmic material.
Discussion
We demonstrated that nuclear LMO2 expression is nearly ubiquitous in the vasculature of native tissues, including lymphatic vasculature, the vasculature of a broad range of neoplasms, and vascular-derived benign and malignant neoplasms. LMO2 reactivity was also identified in breast myoepithelium and in some epithelial-myoepithelial neoplasms, secretory phase endometrium, and a subset of prostatic basal cells. Several salivary gland neoplasms, including pleomorphic adenoma, adenoid cystic carcinoma, and epithelial-myoepithelial carcinoma, have well-documented histologic and immunohistochemical evidence of partial myoepithelial differentiation.11,12 We, therefore, performed immunohistochemical studies for LMO2 in several myoepithelial-derived salivary neoplasms. LMO2 reactivity was seen in the spindled, morphologically myoepithelial component of 2 samples of pleomorphic adenoma and 1 of epithelial-myoepithelial carcinoma, supporting an association of LMO2 with myoepithelial differentiation. The relationship is not absolute, however, as benign salivary gland myoepithelial cells and cutaneous adnexal myoepithelial cells, as well as adenoid cystic carcinoma and myoepithelioma, were LMO2−. We performed a broad survey of carcinomas, sarcomas, and other neoplasms (78 distinct entities) and identified a short list of nonvascular, nonhematolymphoid LMO2+ neoplasms, including giant cell tumor of tendon sheath, GIST, juvenile xanthogranuloma, and some small round blue cell tumors.
Of the vascular beds and vascular-derived neoplasms surveyed, only a small number failed to show LMO2 expression by immunohistochemical studies: hepatic sinusoidal endothelium, myocardial vasculature and endocardium, brain vasculature in older adults, epithelioid vascular malignancies with primary manifestation in the bone and pleura, and infantile hemangioendothelioma of the liver. We demonstrated vascular LMO2 immunoreactivity in gastrointestinal, hematopoietic, genitourinary, and endocrine tissues. Of the native tissues surveyed, only the heart showed uniformly LMO2− vasculature, including the endocardium in fetal and adult specimens. Expression of other surface endothelial markers including CD31, von Willebrand factor (vWF),13 and vascular-endothelial cadherin14 has been demonstrated in myocardial vasculature and endocardium.
Previous studies of expression of LMO2 in brain tissue have been performed in animal models at the level of messenger RNA (mRNA). In developing chick brain, LMO2 mRNA is limited to the vasculature,15 although in adult mice, LMO2 mRNA expression has been demonstrated in specific portions of the thalamus and hippocampus.16 We confirmed that in human cerebral tissue, in fetuses and adults, LMO2 protein expression is largely limited to the vasculature. Rare scattered LMO2+ mononuclear cells, predominantly in areas of inflammation in resection specimens, likely represent inflammatory cells of the monocyte/macrophage lineage (microglia). Although we found that the normal brain vasculature was LMO2+, we demonstrated loss of LMO2 in the brain vasculature of older adults. The loss of LMO2 expression may be a function of aging or may be related to vascular or neurologic disease. The prevalence of cardiovascular comorbidities increases with age, and of the 5 older adults whose brain tissue was examined by immunohistochemical analysis or Western blotting, cardiovascular causes of death were listed for 3 (myocardial infarction, hypertrophic obstructive cardiomyop-athy, and atherosclerosis and hypertension), whereas 2 died of malignancies (glioblastoma multiforme and diffuse large B-cell lymphoma).
It has been previously suggested that LMO2 mRNA and protein is absent in quiescent (resting) native vasculature but up-regulated in tumor vasculature.17 This model was proposed on the basis of mRNA expression studies in transgenic mice and limited immunohistochemical evaluation of paraffin-embedded human tissue (4 lung specimens evaluated with a rabbit polyclonal antibody against a polypeptide derived from the N-terminal domain of LMO2).18 However, our much larger immunohistochemical survey of LMO2 expression in vascular beds showed widespread expression of LMO2 in native tissue vasculature; we additionally confirmed expression of LMO2 protein in selected native tissues (including lung) by immunoblotting.
Expression of LMO2 was, however, lacking in the vasculature of the majority of hepatocellular carcinoma specimens tested (9/10) and in hepatic sinusoidal endothelium. Hepatic sinusoidal endothelium represents a highly specialized fenestrated vascular bed with low to absent expression of multiple endothelial markers such as CD31, CD34, vWF, and vascular-endothelial cadherin.19 The vasculature of hepatocellular carcinomas resembles sinusoidal endothelium at the light microscopic level and, like sinusoidal endothelium, showed weak or absent expression of some endothelial markers such as CD31 and vWF, while others, particularly CD3420 and vascular-endothelial cadherin,21 were up-regulated. The lack of LMO2 reactivity reinforces the similarities between hepatic sinusoidal endothelium and the sinusoid-like vasculature of hepatocellular carcinoma. Only 1 other carcinoma showed loss of LMO2 reactivity in its vasculature in a subset of cases. Approximately one third of clear cell renal cell carcinomas had LMO2− vasculature, a phenomenon that seemed unrelated to Fuhrman grade.
The crisp nuclear localization of LMO2, its consistent expression in vasculature and vascular-derived neoplasms, and its limited staining in other native tissues and nonvascular sarcomas (Table 3) make it an attractive choice for clinical use in diagnostic immunohistochemical analysis. Three existing markers of vascular differentiation, CD31, FLI-1, and CD34, are in widespread clinical use and are commonly used in combination as part of a panel tailored to the differential diagnosis and histologic features of the case. The combination of the highly sensitive and well-known membrane/cytoplasmic marker CD31 and a nuclear marker such as LMO2 or FLI-1 is particularly attractive.
CD31 shows very good sensitivity for vascular neoplasms, including angiosarcomas,22 and, similar to LMO2, is expressed in some lymphoid neoplasms.23 In our series, all vascular neoplasms were CD31+, including the subset of epithelioid vascular malignancies that were LMO2− (Table 2). CD31 may, therefore, be more sensitive than LMO2 overall, although the comparison is difficult to make in a retrospective series when CD31 reactivity was used to make the initial diagnosis. CD31 has been reported to react with occasional carcinomas24,25; in our series, we saw no nuclear LMO2 reactivity in 85 carcinomas surveyed (although 1 of 7 carcinosarcomas was LMO2+). CD31 also reacts with intratumoral histiocytes,26 which can provide false apparent support for vascular differentiation in a poorly differentiated neoplasm. LMO2, by contrast, stains only rare scattered mononuclear cells of probable monocyte/macrophage lineage (specifically, Kupffer cells in the liver, microglia in areas of inflammation in brain resection specimens, and infiltrating mononuclear cells in schwannomas and neurofibromas).
FLI-1 is a transcription factor that was initially identified as a marker of Ewing sarcoma/primitive neuroectodermal tumor and lymphoblastic lymphoma27; like LMO2, FLI-1 demonstrates nuclear reactivity and high sensitivity for malignant vascular neoplasms.28 Commonly, new antibodies that become available to the clinical immunohistochemistry laboratory seem to be very specific; however, their full range of reactivity and limitations are known only with time and experience. The case of FLI-1 is illustrative: initial studies showed high specificity of FLI-1 for Ewing sarcoma/primitive neuroectodermal tumor and lymphoblastic lymphoma27 and vascular neoplasms.28 Later studies29–32 identified FLI-1 immunoreactivity in various types of carcinoma (eg, Merkel cell carcinoma, medullary breast carcinoma, small cell carcinoma of the lung, and urothelial carcinoma); “small round blue cell tumors,” including olfactory neuroblastoma and desmoplastic small round blue cell tumor; various non-Hodgkin lymphomas; and soft tissue neoplasms, including hemangiopericytoma, synovial sarcoma, liposarcoma, and rhabdomyosarcoma. A significant strength of our characterization of LMO2 as a diagnostic marker in nonhematolymphoid neoplasia is the broad range of diagnostic entities that we studied.
CD34 is an effective marker of vascular neoplasms,33 although its sensitivity for angiosarcomas is only moderate22,34 compared with CD31, FLI-1, and LMO2. LMO2 is a more sensitive marker of angiosarcomas than CD34: in our series 25 of 28 angiosarcomas were LMO2+, whereas CD34 was positive in 8 of 15 cases. CD34 is largely negative in poorly differentiated areas of angiosarcoma,35 whereas LMO2 is positive in well-differentiated vasoformative and poorly differentiated angiosarcoma. For example, among epithelioid angiosarcomas, 1 of 6 were CD34+, whereas 4 of 6 were LMO2+. Furthermore, CD34 also reacts with normal dendritic interstitial cells and a wide variety of mesenchymal neoplasms with spindled cell or epithelioid morphologic features such as GIST, solitary fibrous tumor, and epithelioid sarcoma.34,36
Among the malignant vascular neoplasms surveyed, 2 specific entities lacked LMO2 expression: epithelioid hemangioendothelioma of bone and epithelioid angiosarcoma/epithelioid hemangioendothelioma of the pleura. In contrast, 25 of 26 angiosarcomas and 7 of 8 hemangioendotheliomas from a variety of other sites were positive for LMO2. Primary malignant vascular neoplasms of the pleura follow a rapid, aggressive clinical course regardless of their pathologic designation as hemangioendothelioma or angiosarcoma37; epithelioid hemangioendothelioma of bone is often multifocal, with clinical behavior of intermediate malignancy.38 Both entities may be immunoreactive for other markers of vascular differentiation such as CD34, CD31, and vWF,37,39 similar to other vascular malignancies. Thus, the lack of LMO2 immunoreactivity seems to favor a primary site in pleura or bone.
The LMO2 protein seems to be constitutively expressed in most vasculature; its role in angiogenesis may, therefore, be modulated by the expression pattern of its partner in transcriptional activation, the basic helix-loop-helix–containing transcription factor TAL-1/SCL1. LMO2 protein targets specific and distinct DNA sequences in vivo by forming complexes with TAL-1/SCL1.40 Indeed, the complex of TAL-1/SCL1 and LMO2 specifically activates transcription of vascular-endothelial cadherin in endothelial cells.41 TAL-1/SCL1 is absent in quiescent vasculature but is up-regulated in models of physiologic angiogenesis and tumor angiogenesis and lymphangiogenesis.42 Because LMO2 seems to be expressed constitutively in native vasculature, it should be available to form a transcriptionally active complex with TAL-1/SCL1 in proliferative states such as angiogenesis. It is possible that the loss of LMO2 expression in the vasculature of the aging brain may increase susceptibility to ischemic insult. Although LMO2 seems to be constitutively expressed in most native vascular beds, LMO2 expression does seem to be regulated under physiologic circumstances. LMO2 expression in secretory phase but not proliferative phase endometrial glands indicates fairly regular modulation of LMO2 protein levels in half the population and provides a potential model system for understanding regulation of LMO2 expression.
We found that LMO2 expression is ubiquitous in benign vascular neoplasms, including those of blood vascular and lymphovascular derivation, and in the majority of malignant vascular neoplasms. TAL-1/SCL1 has also been shown to be expressed in benign and malignant vascular neoplasms, including hemangioma, Kaposi sarcoma, spindle cell hemangioendothelioma, and angiosarcoma43; it is, therefore, possible that the TAL-1/SCL1 and LMO2 complex is transcriptionally active in vascular neoplasms. Expression of TAL-1/SCL1 has not been assayed in GIST, giant cell tumor of tendon sheath, Ewing sarcoma, or desmoplastic small round blue cell tumor; any possible pathophysiologic role for LMO2 in these tumors remains to be investigated. In the arena of diagnostic pathology, LMO2 has been demonstrated to be a useful prognostic marker in the setting of diffuse large B-cell lymphoma.5,6 It has also been proposed that LMO2 may have a role in prostate cancer progression44; in that study the authors reported strong LMO2 reactivity in 7 of 21 low-grade (Gleason score <7) and 28 of 42 high-grade (Gleason score ≥7) prostate carcinoma specimens. In our study, all 14 prostate cancer specimens (of which 8 were Gleason score ≥7) were negative for nuclear LMO2, with rare cases showing weak cytoplasmic staining of uncertain significance (2 Gleason score 6 and 1 Gleason score 9). The difference may be due to the antibody used: we used a monoclonal antibody, whereas the previous study was performed using a commercially available polyclonal goat antihuman LMO2 antibody. Clearly, this is an area deserving of further study; the possible prognostic significance of LMO2 expression in tumors with partial LMO2 expression, including GIST, Ewing sarcoma, and desmoplastic small round blue cell tumor is also worthy of investigation.
Acknowledgments
We thank Kelli Montgomery and Matt van de Rijn for providing tissue microarrays; Olga Weinberg and John Higgins for help with retrieving archived paraffin-embedded material; and Monique Barakat, Sarah Breitweiser, Erika Dobos, and Mohanpal Dulai for help with obtaining fresh tissue specimens.
Supported by grants CA34233, CA109335, CA33399, and CA122105 from the National Institutes of Health, Bethesda, MD, and the Dwoskin Family Foundation, Miami, FL.
References
- 1.Sanchez-Garcia I, Rabbitts TH. The LIM domain: a new structural motif found in zinc-finger-like proteins. Trends Genet. 1994;10:315–320. doi: 10.1016/0168-9525(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 2.Yamada Y, Warren AJ, Dobson C, et al. The T cell leukemia LIM protein Lmo2 is necessary for adult mouse hematopoiesis. Proc Natl Acad Sci U S A. 1998;95:3890–3895. doi: 10.1073/pnas.95.7.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehm T, Foroni L, Kaneko Y, et al. The rhombotin family of cysteine-rich LIM-domain oncogenes: distinct members are involved in T-cell translocations to human chromosomes 11p15 and 11p13. Proc Natl Acad Sci U S A. 1991;88:4367–4371. doi: 10.1073/pnas.88.10.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 5.Lossos IS, Czerwinski DK, Alizadeh AA, et al. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med. 2004;350:1828–1837. doi: 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- 6.Natkunam Y, Farinha P, Hsi ED, et al. LMO2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy with and without rituximab. J. Clin Oncol. 2008;26:447–454. doi: 10.1200/JCO.2007.13.0690. [DOI] [PubMed] [Google Scholar]
- 7.Yamada Y, Pannell R, Forster A, et al. The oncogenic LIM-only transcription factor Lmo2 regulates angiogenesis but not vasculogenesis in mice. Proc Natl Acad Sci U S A. 2000;97:320–324. doi: 10.1073/pnas.97.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Natkunam Y, Zhao S, Mason DY, et al. The oncoprotein LMO2 is expressed in normal germinal-center B cells and in human B-cell lymphomas. Blood. 2007;109:1636–1642. doi: 10.1182/blood-2006-08-039024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Natkunam Y, Warnke RA, Montgomery K, et al. Analysis of MUM1/IRF4 protein expression using tissue microarrays and immunohistochemistry. Mod Pathol. 2001;14:686–694. doi: 10.1038/modpathol.3880373. [DOI] [PubMed] [Google Scholar]
- 10.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 11.Savera AT, Gown AM, Zarbo RJ. Immunolocalization of three novel smooth muscle–specific proteins in salivary gland pleomorphic adenoma: assessment of the morphogenetic role of myoepithelium. Mod Pathol. 1997;10:1093–1100. [PubMed] [Google Scholar]
- 12.Prasad AR, Savera AT, Gown AM, et al. The myoepithelial immunophenotype in 135 benign and malignant salivary gland tumors other than pleomorphic adenoma. Arch Pathol Lab Med. 1999;123:801–806. doi: 10.5858/1999-123-0801-TMIIBA. [DOI] [PubMed] [Google Scholar]
- 13.Taylor PM, Rose ML, Yacoub MH, et al. Induction of vascular adhesion molecules during rejection of human cardiac allografts. Transplantation. 1992;54:451–457. doi: 10.1097/00007890-199209000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Breier G, Breviario F, Caveda L, et al. Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood. 1996;87:630–641. [PubMed] [Google Scholar]
- 15.Herberth B, Minkó K, Csillag A, et al. SCL, GATA-2 and Lmo2 expression in neurogenesis. Int J Dev Neurosci. 2005;23:449–463. doi: 10.1016/j.ijdevneu.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Hinks GL, Shah B, French SJ, et al. Expression of LIM protein genes Lmo1, Lmo2, and Lmo3 in adult mouse hippocampus and other forebrain regions: differential regulation by seizure activity. J Neurosci. 1997;17:5549–5559. doi: 10.1523/JNEUROSCI.17-14-05549.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada Y, Pannell R, Forster A, et al. The LIM-domain protein Lmo2 is a key regulator of tumour angiogenesis: a new anti-angiogenesis drug target. Oncogene. 2002;21:1309–1315. doi: 10.1038/sj.onc.1205285. [DOI] [PubMed] [Google Scholar]
- 18.Warren AJ, Colledge WH, Carlton MB, et al. The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell. 1994;78:45–57. doi: 10.1016/0092-8674(94)90571-1. [DOI] [PubMed] [Google Scholar]
- 19.Lalor PF, Lai WK, Curbishley SM, et al. Human hepatic sinusoidal endothelial cells can be distinguished by expression of phenotypic markers related to their specialised functions in vivo. World J Gastroenterol. 2006;12:5429–5439. doi: 10.3748/wjg.v12.i34.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruck P, Xiao JC, Kaiserling E. Immunoreactivity of sinusoids in hepatocellular carcinoma: an immunohistochemical study using lectin UEA-1 and antibodies against endothelial markers, including CD34. Arch Pathol Lab Med. 1995;119:173–178. [PubMed] [Google Scholar]
- 21.Kato K, Takada T, Fukusato T. Expression of vascular endothelial-cadherin in human hepatocellular carcinoma tissues. Hepatol Res. 2007;37:444–453. doi: 10.1111/j.1872-034X.2007.00051.x. [DOI] [PubMed] [Google Scholar]
- 22.Miettinen M, Lindenmayer AE, Chaubal A. Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens: evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand factor. Mod Pathol. 1994;7:82–90. [PubMed] [Google Scholar]
- 23.Nicholson SA, McDermott MB, DeYoung BR, et al. CD31 immunoreactivity in small round cell tumors. Appl Immunohistochem Mol Morphol. 2000;8:19–24. doi: 10.1097/00129039-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 24.De Young BR, Frierson HF, Jr, Ly MN, et al. CD31 immunoreactivity in carcinomas and mesotheliomas. Am J Clin Pathol. 1998;110:374–377. doi: 10.1093/ajcp/110.3.374. [DOI] [PubMed] [Google Scholar]
- 25.Sapino A, Bongiovanni M, Cassoni P, et al. Expression of CD31 by cells of extensive ductal in situ and invasive carcinomas of the breast. J Pathol. 2001;194:254–261. doi: 10.1002/1096-9896(200106)194:2<254::AID-PATH880>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 26.McKenney JK, Weiss SW, Folpe AL. CD31 expression in intratumoral macrophages: a potential diagnostic pitfall. Am J Surg Pathol. 2001;25:1167–1173. doi: 10.1097/00000478-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Folpe AL, Hill CE, Parham DM, et al. Immunohistochemical detection of FLI-1 protein expression: a study of 132 round cell tumors with emphasis on CD99-positive mimics of Ewing’s sarcoma/primitive neuroectodermal tumor. Am J Surg Pathol. 2000;24:1657–1662. doi: 10.1097/00000478-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Folpe AL, Chand EM, Goldblum JR, et al. Expression of Fli-1, a nuclear transcription factor, distinguishes vascular neoplasms from potential mimics. Am J Surg Pathol. 2001;25:1061–1066. doi: 10.1097/00000478-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Rossi S, Orvieto E, Furlanetto A, et al. Utility of the immunohistochemical detection of FLI-1 expression in round cell and vascular neoplasm using a monoclonal antibody. Mod Pathol. 2004;17:547–552. doi: 10.1038/modpathol.3800065. [DOI] [PubMed] [Google Scholar]
- 30.Llombart B, Monteagudo C, Lopez-Guerrero JA, et al. Clinicopathological and immunohistochemical analysis of 20 cases of Merkel cell carcinoma in search of prognostic markers. Histopathology. 2005;46:622–634. doi: 10.1111/j.1365-2559.2005.02158.x. [DOI] [PubMed] [Google Scholar]
- 31.Tiemann K, Heitling U, Kosmahl M, et al. Solid pseudopapillary neoplasms of the pancreas show an interruption of the Wnt-signaling pathway and express gene products of 11q. Mod Pathol. 2007;20:955–960. doi: 10.1038/modpathol.3800902. [DOI] [PubMed] [Google Scholar]
- 32.Mhawech-Fauceglia P, Herrmann FR, Bshara W, et al. Friend leukaemia integration-1 expression in malignant and benign tumours: a multiple tumour tissue microarray analysis using polyclonal antibody. J Clin Pathol. 2007;60:694–700. doi: 10.1136/jcp.2006.039230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traweek ST, Kandalaft PL, Mehta P, et al. The human hematopoietic progenitor cell antigen (CD34) in vascular neoplasia. Am J Clin Pathol. 1991;96:25–31. doi: 10.1093/ajcp/96.1.25. [DOI] [PubMed] [Google Scholar]
- 34.van de Rijn M, Rouse RV. CD34: a review. Appl Immunohistochem. 1994;2:71–80. [Google Scholar]
- 35.Poblet E, Gonzalez-Palacios F, Jimenez FJ. Different immunoreactivity of endothelial markers in well and poorly differentiated areas of angiosarcomas. Virchows Arch. 1996;428:217–221. doi: 10.1007/BF00196693. [DOI] [PubMed] [Google Scholar]
- 36.Natkunam Y, Rouse RV, Zhu S, et al. Immunoblot analysis of CD34 expression in histologically diverse neoplasms. Am J Pathol. 2000;156:21–27. doi: 10.1016/S0002-9440(10)64701-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang PJ, Livolsi VA, Brooks JJ. Malignant epithelioid vascular tumors of the pleura: report of a series and literature review. Hum Pathol. 2000;31:29–34. doi: 10.1016/s0046-8177(00)80194-x. [DOI] [PubMed] [Google Scholar]
- 38.O’Connell JX, Nielsen GP, Rosenberg AE. Epithelioid vascular tumors of bone: a review and proposal of a classification scheme. Adv Anat Pathol. 2001;8:74–82. doi: 10.1097/00125480-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Kulkarni KR, Jambhekar NA. Epithelioid hemangioendothelioma of bone: a clinicopathologic and immunohistochemical study of 7 cases. Indian J Pathol Microbiol. 2003;46:600–604. [PubMed] [Google Scholar]
- 40.Grutz GG, Bucher K, Lavenir I, et al. The oncogenic T cell LIM-protein Lmo2 forms part of a DNA-binding complex specifically in immature T cells. EMBO J. 1998;17:4594–4605. doi: 10.1093/emboj/17.16.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deleuze V, Chalhoub E, El-Hajj R, et al. TAL-1/SCL and its partners E47 and LMO2 up-regulate VE-cadherin expression in endothelial cells. Mol Cell Biol. 2007;27:2687–2697. doi: 10.1128/MCB.00493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang T, Shi Y, Opalenik SR, et al. Expression of the TAL1/SCL transcription factor in physiological and pathological vascular processes. J Pathol. 2006;210:121–129. doi: 10.1002/path.2028. [DOI] [PubMed] [Google Scholar]
- 43.Chetty R, Dada MA, Boshoff CH, et al. TAL-1 protein expression in vascular lesions. J Pathol. 1997;181:311–315. doi: 10.1002/(SICI)1096-9896(199703)181:3<311::AID-PATH775>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 44.Ma S, Guan XY, Beh PS, et al. The significance of LMO2 expression in the progression of prostate cancer. J Pathol. 2007;211:278–285. doi: 10.1002/path.2109. [DOI] [PubMed] [Google Scholar]