Abstract
Antiviral CD8+ T cell recognition of MHC Class I-peptide complexes on the surface of professional APCs is a requisite step in an effective immune response following many potentially lethal infections. Although MHC Class I-peptide production is thought to be closely linked to the continued presence of virus, several studies have shown that the persistence of antigen presentation occurs for an extended period of time following the clearance of RNA viruses. However, the mechanism responsible for antigen presentation persistence following viral clearance was unknown until now. Here, we utilized a recombinant DNA virus expressing different forms of a model antigen to study the mechanism of prolonged antigen presentation in mice. We determined that the persistence of antigen presentation consists of three distinct mechanistic phases: ongoing viral replication, persistence of virally infected cells, and cross presentation of antigen. These data will allow manipulation of the form of antigen contained within viral vectors to produce the most effective and protective CD8+ T cell response to be generated following vaccination.
Introduction
CD8+ T cells (TCD8+) play a crucial role in immunity to viruses. Antiviral TCD8+ are initially activated by recognition of MHC Class I-peptide (pMHC-I) complexes on the surface of professional APCs (pAPC), but recognition of pMHC-I complexes on pAPC is also likely required for efficient activation of memory TCD8+ (1, 2). Antigen presentation of pMHC-I by pAPC is generally held to be down-regulated before the clearance of antigen or bacterial pathogen (3–5). However, several studies have shown that the persistence of antigen presentation occurs for an extended period of time following clearance of RNA viruses that cause acute, but not persistent, infection (6–8). The mechanisms responsible for continued antigen presentation following clearance of detectable levels of virus remain unknown.
Generation of pMHC-I by pAPC can occur via at least two physically and mechanistically distinct presentation pathways, direct or cross presentation. In the case of a virus infection, direct presentation occurs from any cells that are infected with virus, and peptides conjugated to MHC Class I are generated efficiently from short-lived protein substrates that may be incorrectly folded or translated (9, 10). In contrast, cross presentation is the internalization of proteinaceous material from virus infected cells by uninfected pAPC, and generally involves the transfer of longer-lived antigenic substrates (11–13). Exogenous antigen was retained in DC for days, potentially implicating cross-presentation of antigen in the prolonging of antigen presentation (14). Here we utilized a recombinant antigen, ovalbumin (OVA), expressed in a form that can be presented by both the cross and direct presentation pathways (OVA full-length [FL]). We compared OVA-FL to an antigenic form (OVA mini-gene [MG]) that multiple independent laboratories (12, 13, 15, 16) have demonstrated is restricted exclusively to the direct presentation pathway, likely because the half-life of this form of antigen is too short to facilitate transfer to another cell without additional stabilization (17). Although a small number of minimal antigenic peptides can be cross presented the OVA peptide studied here is completely restricted to the direct presentation pathway in vivo (18). By comparing the activation of naïve antigen-specific T cells following infection with recombinant viruses we were able to examine the contribution of direct and cross presentation to the persistence of antigen presentation.
We examined persistence of antigen following infection with recombinant vaccinia virus (rVACV), a DNA virus that is unlikely to integrate its nucleic acids into infected cells as it is highly cytotoxic and replicates wholly in the cytosol of infected cells. Replicating VACV can only be detected for 2 weeks post infection, but activation of adoptively transferred naïve TCD8+ can be detected for 40+ days after infection. After detectable levels of virus are cleared direct presentation persists, implying the existence of virus-infected cells for this period. A final phase of antigen presentation involves cross presentation of antigen. The data yielded here will allow manipulation of the form of antigen contained within viral vectors or other vaccine preparations to allow the presentation of antigen for different periods of time, allowing the most effective and protective TCD8+ response to be generated following vaccination.
Materials and Methods
Mice
C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). OT-1 TCR RAG1−/− (19, 20) transgenic mice were obtained from the NIAID Exchange Program (Line 4175). Where indicated, OT-1 mice were bred to B6.SJL mice (Taconic). MAFIA mice (21) were purchased from The Jackson Laboratory. CD11cDTR/GFP mice (22) were purchased from Jackson Laboratory and subsequently backcrossed to C57BL/6 mice to achieve N10. All mice were maintained under specific pathogen-free conditions in the animal facility at the Pennsylvania State M.S. Hershey College of Medicine. All experiments and breeding were approved by the Penn State College of Medicine Institutional Animal Care and Use Committee.
Viruses
Recombinant vaccinia viruses expressing ovalbumin and minigene were provided by Drs. Jon Yewdell and Jack Bennink (National Institutes of Health, Bethesda, MD). Female C57BL/6 mice (6–10 wks-old) were injected intravenously (i.v.) with 106 pfu rVACV. Virus was titrated from tissue after three freeze/thaw cycles. For Real Time PCR analyses tissue was homogenized and RNA was purified using the RNeasy Mini Kit (spleens) or RNeasy Plus Mini Kit (ovaries). After measuring RNA concentration and purity, we converted mRNA into cDNA and used a Bio-Rad iQ Powermix and an ABI 9700HT or Bio-Rad Opticon 2 to detect full-length OVA cDNA. For OVA PCR, we used the protocol and primer concentrations from Klochkov et al (23) and primers were as follows; OVA forward: 5’-GCA GCA CAT GCA GAA ATC A-3’, OVA reverse: 5’-AGA AGA GAA CGG CGT TGG T-3’, OVA probe: (FAM)-5’-CAT TCC TCT TCT GTA TCA AGC ACA TCG-3’-(BHQ). As positive controls and standard curves pRB21-eGFP-OVA-FL plasmid (24) was used for OVA PCR. This assay could reliably detect 10 copies of the sequence in question. Similar results were obtained using a PCR assay to detect VACV E9L cDNA.
Cell lines and cultures
All media were purchased from Invitrogen (Grand Island, NY). Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 10% FBS, antibiotics (penicillin and streptomycin) and 2 mM glutamine was used during effector assays and for adoptive transfers where shown. β2M−/− cells (13) were maintained in Dulbecco’s Modified Eagle Media containing 10% fetal bovine serum (FBS) supplemented with penicillin/streptomycin and 2mM L-glutamine.
UV/psoralen treatment
To treat virus stocks 4,5',8-Trimethylpsoralen (Psoralen, Sigma) was added to 1 ml rVACV stock (1×108 p.f.u./ml−1) to give a final concentration of 10 µg/ml. The stock was then exposed to UVA (295nm) for times indicated by placing a portable UV lamp approximately 1 cm above the liquid (25). Virus exposed to UVA for greater than 10 minutes always failed to produce any plaques in a standard titration. To treat virus-infected cells, psoralen was added to β2M−/− cells infected with virus for 5h to give a final concentration of 10 µg/ml−1. Cells were exposed to UVC (254 nm) as described with mechanical agitation every 5 minutes. Prior to exposure to UVC and psoralen approximately 5 × 106 pfu could be liberated from 5 × 106 infected cells previously exposed to VACV at a multiplicity of infection of 10, but after exposure to UVC and psoralen this was reduced to undetectable levels, indicating full ablation of VACV replication by this treatment.
In vivo cross-presentation
For in vivo infection, mice were injected i.p. with 5 × 106 β2M−/− fibroblasts that were infected with VACV-OVA at a multiplicity of infection of 10 for 5h, then treated with psoralen and ultraviolet light (UVC), and gamma irradiated at 20,000 rads as previously described (13). Greater than 80% of β2M−/− fibroblasts were infected and expressing detectable viral antigen following UVC and psoralen treatment. Injection of CPG oligonucleotides or LPS 12 h prior to injection of β2M−/− fibroblasts completely ablated proliferation of adoptively transferred TCD8+, indicating that cross presentation was the pathway of antigen presentation used (26).
Adoptive Transfer of TCR Transgenic T cells
T cell enriched populations were obtained from T cell receptor (TCR) transgenic (TCR Tg) mice as follows. Lymph nodes (popliteal, inguinal, brachial, axillary and superficial cervical) and spleens were harvested and a single-cell suspension generated using a homogenizer. Live cells were isolated by centrifugation over lymphocyte separation medium (LSM). Cells were washed, resuspended and 1–5 × 106 cells were injected into each recipient via the lateral tail vein using a 27 gauge needle.
Analysis of cell division in vivo
To analyze cell division in vivo, adoptively transferred TCR Tg T cells were labeled with the dye 5-(and-6) carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE, Molecular Probes, Eugene, OR). Mononuclear cells isolated over an LSM gradient were washed and then labeled with 5mM CFDA-SE for 10 min at 37°C washed and injected i.v. into recipient mice. Primary lymphocytes were harvested at the times indicated following infection and analyzed for cell division as indicated by dilution of the CFDA-SE dye.
Three days after injection, spleens were harvested and homogenized. Cells were harvested from the spleen as infections were performed IV, and so the spleen is the most relevant site at which to examine T cell proliferation. In addition, by examining proliferation 3 days after injection there is sufficient time for proliferating cells to circulate systemically frsom sites of activation to the spleen. Mononuclear splenocytes were isolated by centrifugation over LSM. Cells were incubated in 2.4G2 (27) supernatant/20% normal mouse serum for 20 min on ice to block Fc receptor-mediated binding of antibody, then stained with anti-CD8-PE Cy5 (Clone 53-6.7) and anti-CD45.1-PE (Clone A20) for 40 min on ice. Only cells expressing CD8 and CD45.1 were analyzed for CFDA-SE staining.
Depletion of Cells In Vivo
For systemic DC depletion, CD11c-DTR/GFP transgenic mice were injected intraperitoneally with 4 ng/g body weight diphtheria toxin (DT) (in PBS; Sigma Chemical Co., St. Louis, MO, USA) (22). To deplete phagocytes mice were injected with 200 µl of clodronate liposomes in PBS i.v. 1 day prior to injection of TCD8+ and every day after until sacrifice. This injection scheme effectively depletes monocytes and many macrophage populations but maintains DC populations during VACV infection (Fig. 6B). Cl2MDP (or clodronate) was from Roche Diagnostics GmbH, Mannheim, Germany. Liposomes were prepared using Phosphatidylcholine (LIPOID E PC, Lipoid GmbH) and cholesterol (Sigma).
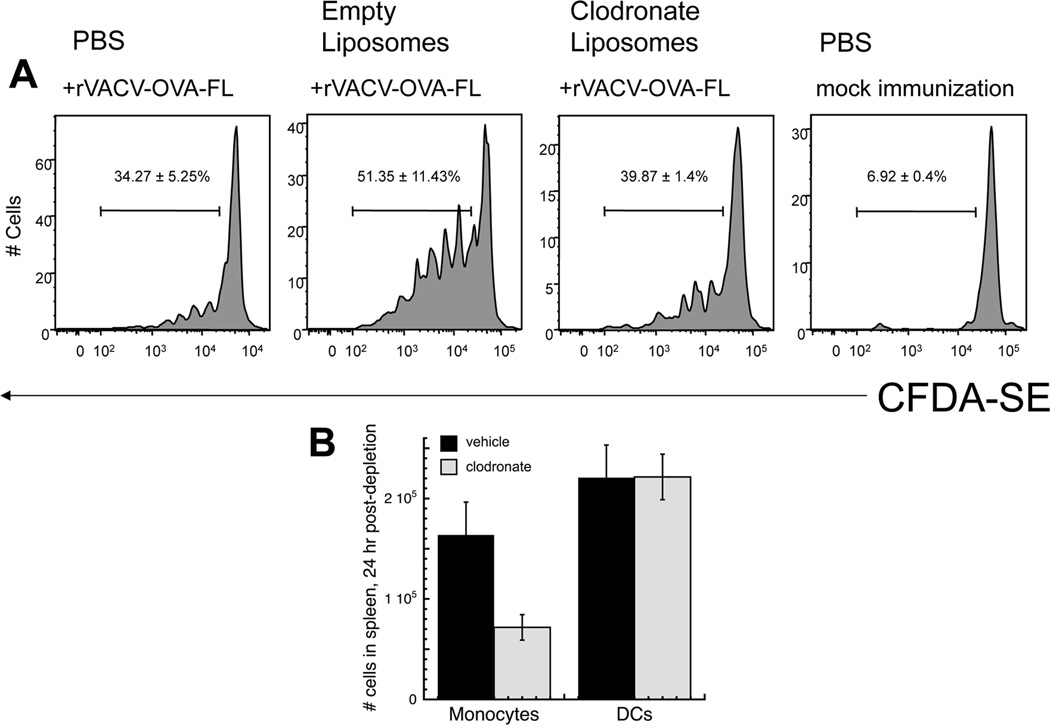
Figure 6. Depletion of phagocytes failed to prevent antigen presentation persistence during the phase of persistently infected cells.
(A) - C57BL/6 mice were infected with rVACV expressing OVA FL. 20 days post infection mice were treated with 200µl of clodronate liposomes, empty liposomes or PBS i.v.. One day after treatment, OT-1.SJL T cells were labeled with CFDA-SE and transferred i.v. into previously infected mice. 3 days after transfer, spleens were removed and analyzed for CFDA-SE dilution. Representative histograms display gated CD45.1+ TCD8+ from individual mice of replicate experiments using 4 mice per condition. Gates represent the percentage of CD45.1+ TCD8+ cells that proliferated. Numbers represent the standard error of percent CD45.1+ TCD8+ proliferation. (B) - Mice were infected with 106 pfu rVACV and 15 days post-infection, VACV-infected mice were injected with clodronate liposomes (250 ul) or PBS (i.v.). Spleens were isolated 24 hours later, and single-cell suspensions of splenocytes were stained for monocyte/macrophages (F4/80+, CD11c−) and dendritic cells (CD11c+ F4/80−). Data represent the mean and SEM of at least 4 individual mice.
Ex vivo stimulation and ICS assay
B6.SJL (CD45.1+) mice were infected i.v. with 106 PFU of rVACV-OVA FL. On d21 post-infection, single cell suspensions were prepared from lymph nodes and spleens of OT-I TCR Tg × Rag1−/− mice followed by isolation of live cells as described above. Frequency of CD8α+ Vβ5.1 TCR+ cells was determined by flow cytometry. 106 Vβ5.1+ OT-I TCR Tg TCD8+ cells were then transferred i.v. into the infected mice.
7d post-transfer, spleens were harvested and single cell suspensions prepared. 2×106 cells per sample were stimulated for 5 hrs with 1 µg/mL of SIINFEKL peptide in the presence of 10 µg/mL of brefeldin A (Sigma-Aldrich, St. Louis, MO.) at 37°C, 5% CO2. After stimulation, Fc receptors were blocked with 2.4G2 supernatant/10% normal mouse serum prior to staining with PerCP-Cy5.5-conjugated anti-CD45.1 and V450-conjugated anti-CD8α. Cells were then fixed for 20 minutes at room temperature in 2% paraformaldehyde and permeabilized in 2.4G2 supernatant/10% mouse serum containing 0.5% saponin for 15 minutes on ice. Cells were then stained with allophycocyanin-conjugated anti-mouse IFN-γ (Clone XMG1.2) in permeabilization buffer for 30 minutes on ice. Finally, cells were washed 3 times in 1× PBS/2% FCS/0.5% saponin and twice in HBSS/0.1% BSA.
Flow cytometric analysis
Cells were washed thoroughly prior to analysis (typically a minimum of 3 washes), then data were acquired using either a FACScan®, FACSCalibur®, or FACSCanto® flow cytometer (Becton Dickinson, San Jose, CA) and analyzed using FlowJo software (Treestar, San Carlos, CA).
Results
In order to study the mechanism by which antigen is able to persist following an acute virus infection it was essential to fully document the presence of virus in infected mice. We examined the presence of rVACV, a large cytotoxic DNA virus that replicates cytosolically and is not known to incorporate nucleic acids into the genome of infected cells. We utilized rVACV encoding the recombinant antigen OVA (rVACV-OVA FL). We infected mice with rVACV and titered virus from the primary site of replication, the ovaries. Titration is more sensitive in our hands than examination of the presence of individual VACV proteins or reporter genes such as GFP or β-Galactosidase. Replicating rVACV was detectable for 12–14 days post infection, but was never detected after d15 post infection (Fig. 1A). We have previously published that VACV is cleared rapidly from secondary lymphoid organs following peripheral or systemic infection (28) but to ensure that this was the case here we titered virus from spleen following i.v. infection. Even when we removed red blood cells to increase the sensitivity of the assay by removing potentially cytotoxic effects on the monolayer used during titration we could never find replicating VACV at d14 post infection (Fig. S1). A plaque assay measures the presence of replicating virus. However, to extend this observation, we assayed the presence of viral RNA from the ovaries or spleen of infected mice by Real Time quantitative PCR (qPCR). The probes we used were specific for either the E9L DNA polymerase, a clinically validated assay used to measure the presence of multiple poxviruses, or for OVA, the antigen used in our presentation assays. Our results using qPCR were similar to those found with the plaque assay, as no detectable signal was present later than day 14 post infection (Fig. S2).
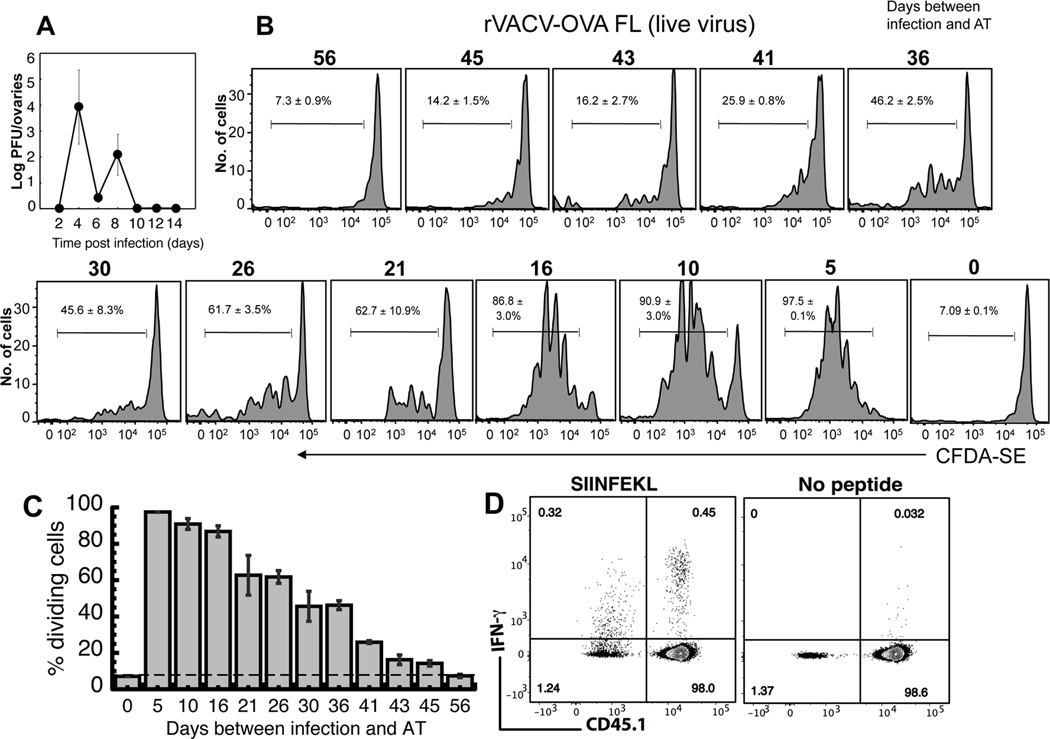
Figure 1. The persistence of virus and antigen presentation following infection with rVACV.
(A) Mice were infected with rVACV and ovaries were removed at times indicated. A plaque assay was used to determine rVACV titer. Error bars represent the standard error. (B) OT-1.SJL TCD8+ cells were labeled with CFDA-SE and transferred i.v. into mice previously infected with rVACV-OVA FL. Three days after transfer, spleens were removed and analyzed for CFDA-SE dilution. Representative histograms display gated CD45.1+ TCD8+ from individual mice of five replicate experiments with similar results. Numbers represent the mean percentage of CD45.1+ TCD8+ that have undergone proliferation. (C) Graphical representation of the percentage of replicating OT-1.SJL TCD8+ cells at each time point after VACV infection. Error bars represent the standard error. (D) OT-1 TCD8+ cells (CD45.2) were transferred i.v. into B6.SJL (CD45.1+) mice infected with rVACV-OVA.FL 21 d previously. On d7 post-transfer, spleens were harvested and cells stimulated ex vivo for 5 hrs with SIINFEKL in the presence of Brefeldin A. IFN-γ production by donor (CD45.1−) and recipient (CD45.1+) TCD8+ cells was assessed by intracellular cytokine staining assay. Flow plots are gated on CD8α+ cells and are representative of 2 independent experiments with 4 mice each.
To determine the persistence of antigen presentation following infection with rVACV we infected mice and adoptively transferred OVA-specific OT-I TCD8+, which are specific for the OVA257–264 peptide presented on MHC Class I, at various times post infection. We used this methodology as the only alternative, ex vivo culture of pAPC populations with naïve TCD8+, is much less sensitive (29). We labeled the OT-I TCD8+ with CFDA-SE and transferred them into the previously infected mice and measured TCD8+ proliferation 3 days later. We used proliferation as the most sensitive measure of antigen presentation as a functional assay of T cell activation may be biased by the phenotype and activation status of the APC, with some APC inducing T cell proliferation, but functional unresponsiveness, following antigen presentation. We were able to detect TCD8+ proliferation of cells in the spleen for over 40 days post infection (Fig. 1B, C) although the proliferation observed did decline significantly between 16 and 21 days post infection in multiple experiments. As expected after an i.v. infection, the proliferation observed in the spleen was always greater than that observed at any other site, including lymph nodes or bone marrow (not shown). To explore whether the proliferation of TCD8+ was accompanied by an acquisition of effector function we adoptively transferred OT1 TCD8+ (CD45.2) into B6.SJL mice (CD45.1) 21d after infection to allow us to distinguish cells that had been primed during the initial infection and those that were primed after the clearance of virus to below detectable levels. 7d later splenocytes were harvested and production of IFN-γ in response to the OVA257–264 peptide was measured. Figure 1D demonstrates that although levels of IFN-γ produced by cells primed exclusively during the period of antigen persistence were lower than those primed during the initial infection, IFN-γ was produced at levels higher than background. Thus, while the greatest proliferation occurred during the time when virus was still detectable by viral titers, antigen presentation capable of triggering naïve TCD8+ to proliferate and acquire effector function still occurred for weeks following viral clearance.
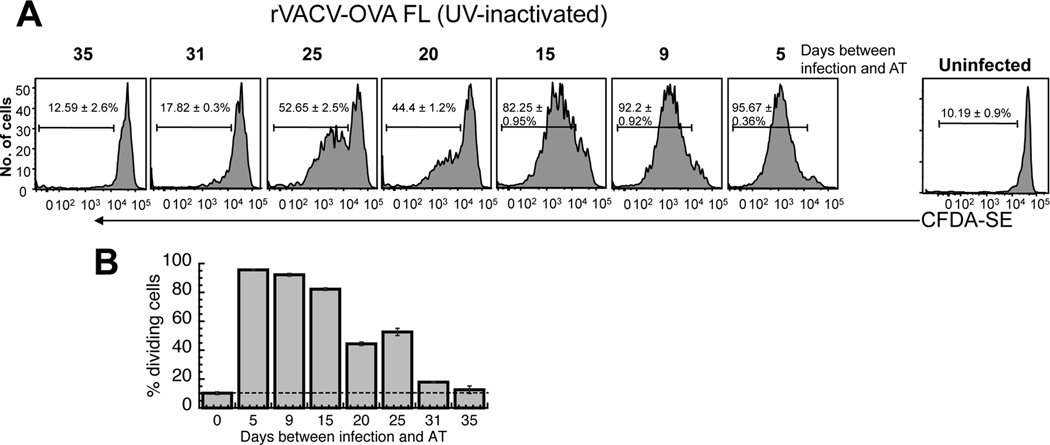
Although we used multiple assays to characterize the clearance of VACV from infected mice, it is not formally possible to exclude the presence of low levels of virus that are present below the level of detection of our assays, or in obscure tissues which we did not examine for the presence of virus. Therefore to assess the role of virus replication in the prolonged antigen presentation we treated stocks of rVACV with UVA and the DNA intercalating agent psoralen to render the virus replication deficient. This is a method we have previously used extensively (13, 25), and we ensured that each batch of virus produced following treatment is not replication competent. Treatment with UVA and psoralen for 20 minutes allowed production of early proteins while preventing production of late proteins and thus virus replication (30) (Fig. S3). We infected mice i.v. with UVA/psoralen treated rVACV-OVA FL at the times indicated. TCD8+ proliferation was detectable for approximately 30 days post infection with replication deficient rVACV (Fig. 2A, B), as compared to approximately 45 days following infection with replicating rVACV (Fig. 1B). Interestingly, this reduction in the duration of antigen presentation (approx. 15 days) closely corresponded to the time period that we were able to detect replicating virus by viral titers (Fig. 1A). The reduced duration of antigen presentation was not due to reduced antigen load upon infection, as equivalent levels of antigen driven by the early promoter driving OVA were produced upon UVA/psoralen exposure (Fig S3) and equivalent numbers of cells were infected by treated or untreated VACV stocks in previous studies (25). Therefore we can accurately account for the requirement for replication in continued antigen presentation and surprisingly, VACV replication is essential for only around a third of the time during which antigen presentation occurs.
Figure 2. Requirement of virus replication for prolonged antigen presentation.
rVACV was inactivated with a 20 minute treatment with UVA/psoralen. OT-1.SJL T cells were labeled with CFDA-SE and transferred i.v. into mice previously infected with rVACV-OVA FL. Three days after transfer, spleens were removed and analyzed for CFDA-SE dilution. Representative histograms display gated CD45.1+ TCD8+ from individual mice of replicate experiments using 8 mice per condition. Gates represent the percentage of CD45.1+ TCD8+ cells that proliferated. Numbers represent the mean percentage of CD45.1+ TCD8+ that have undergone proliferation. (B) Graphical representation of the percentage of replicating OT-1.SJL TCD8+ cells at each time point after VACV infection. Error bars represent the standard error.
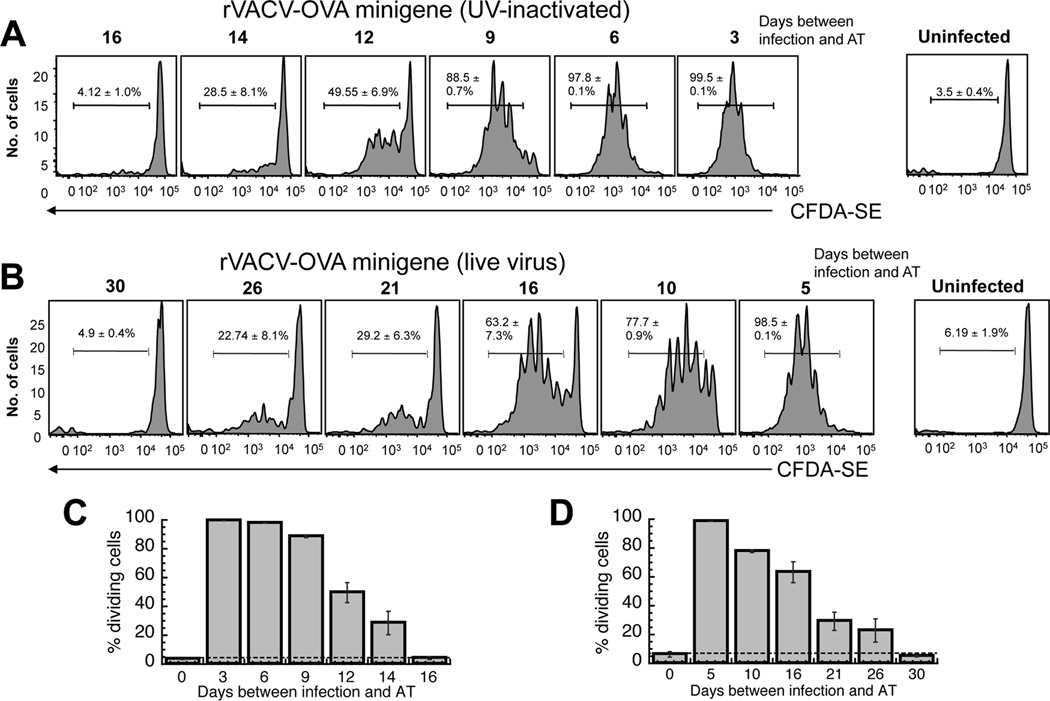
Thus far, we showed that virus replication was required for 12–14 of the 40+ days of antigen presentation persistence. However, approximately 30 days of antigen presentation was not explained by this mechanism. It is possible that persistently infected cells were directly presenting antigen, or that RNA or DNA had been transferred from infected cells to uninfected cells and could result in continued production of antigen for up to 30 days. To study the mechanism of persistent antigen presentation following cessation of virus replication we utilized rVACV expressing a “minigene” (MG) the minimal antigenic determinant from OVA, residues 257–264 (SIINFEKL). rVACV expressing OVA MG has a short half-life of 7 seconds and can only be directly presented by infected cells (17). We treated rVACV-OVA MG with UVA and psoralen, a treatment that does not alter generation of the p-MHC I complex (25) and examined proliferation of OVA-specific TCD8+ at various times after infection. Antigen-specific proliferation of TCD8+ returned to background levels between 14 and 16 days post infection (Fig. 3A, C), indicating that direct presentation by infected cells, or cells that have obtained nucleic acids encoding antigen, persists for around 2 weeks following an initial infection. As virus replication can be detected for around 14 days we would expect that the duration of antigen presentation following infection with replicating rVACV-OVA MG would be the period of replication (~ 14 days) plus the period of direct presentation following infection (~14 days) to give a total of 28 days. Upon infection with replicating rVACV-OVA MG levels of proliferation of adoptively transferred OT-I returned to background levels following adoptive transfer between 26 and 30 days post infection (Fig. 3B, D), as we had predicted.
Figure 3. The requirement for presentation by rVACV infected cells in prolonged antigen presentation.
C57BL/6 mice were infected with non-replicating (A) or replicating (B) rVACV-OVA MG at times indicated. Virus replication was blocked with a 20 minute treatment with UVA/psoralen. OT-1.SJL T cells were labeled with CFDA-SE and transferred i.v. into previously infected mice. 3 days after transfer, spleens were removed and analyzed for CFDA-SE dilution. Representative histograms display gated CD45.1+ TCD8+ from individual mice of replicate experiments using 6 mice per condition. Gates represent the percentage of CD45.1+ TCD8+ cells that proliferated. (C, D) Graphical representation of the percentage of replicating OT-1.SJL TCD8+ cells at each time point after VACV infection in A and B respectively. Error bars represent the standard error.
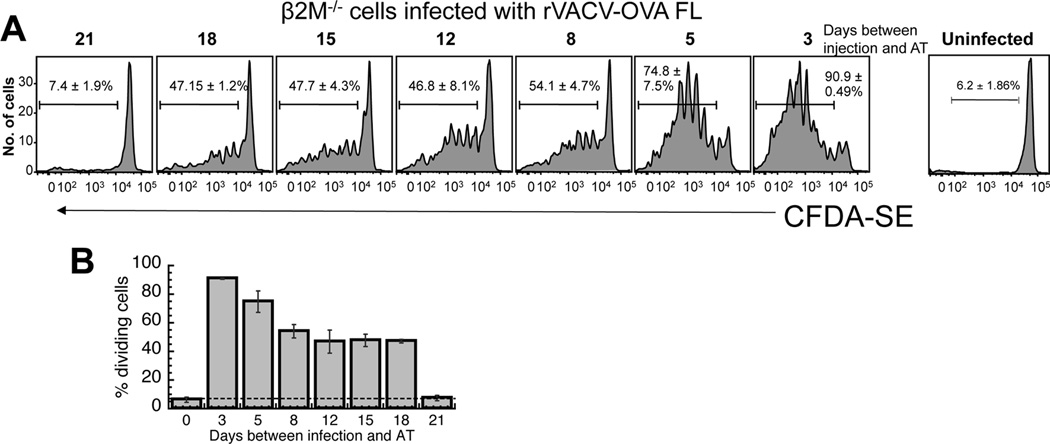
To this point we had been able to account for 26–30 days of the 45-day period for which antigen presentation occurs following infection with rVACV-OVA. Full length OVA has a half-life of around 280 minutes (24) and is available for cross presentation (12, 13) as well as direct presentation by VACV infected cells. However, OVA MG can only be directly presented by infected cells (13), raising the intriguing possibility that the deficit of 18 days of antigen presentation we observed after infection with rVACV-OVA MG vs. OVA FL was due to a lack of cross presentation (Fig. 3B, D). To examine the contribution of cross presentation in the persistence of antigen presentation, we used cells that do not express β2 microglobulin (β2M−/− cells) and are unable to directly present antigen. We have been unable to directly detect antigen transfer from injected cells to host cells over many years of experimentation, so we again assayed TCD8+ proliferation, which in this case can only occur via cross presentation of antigen following immunization with these cells. β2M−/− cells were infected for 5 hr to produce levels of antigen greater than or equal to levels of antigen found in cells ex vivo following infection with replication competent VACV. Mice were immunized with 5 × 106 β2M−/− cells, of which >80% were detectably infected, to produce a similar number of infected cells, and similar antigen load, to immunization with 5 × 106 pfu of non-replicating VACV. When mice were immunized with β2M−/− cells infected with rVACV-OVA FL, we were able to detect TCD8+ proliferation for approximately 18 days (Fig. 4A, B). This was the exact difference in the number of days between mice infected with rVACV-OVA MG (direct presentation – 26 days) and OVA FL (direct and cross presentation – 45 days). Thus, cross presentation of antigen accounted for the remaining days of antigen presentation in our system.
Figure 4. Prolonged presentation of cell-derived antigen (cross presentation).
Mice were immunized with β2 microglobulin knockout (β2M−/−) cells infected with rVACV-OVA FL for 5 hours at times indicated. To prevent viral replication, cells were then treated with UVC/psoralen for 20 minutes. OT-1.SJL T cells were labeled with CFDA-SE and transferred i.v. into previously immunized mice. 3 days after transfer, spleens were removed and analyzed for CFDA-SE dilution. Representative histograms display gated CD45.1+ TCD8+ from individual mice of replicate experiments using 8 mice per condition. Gates represent the percentage of CD45.1+ TCD8+ cells that proliferated. Numbers represent the standard error of percent CD45.1+ TCD8+ proliferation. (B) Graphical representation of the percentage of replicating OT-1.SJL TCD8+ cells at each time point after VACV infection. Error bars represent the standard error.
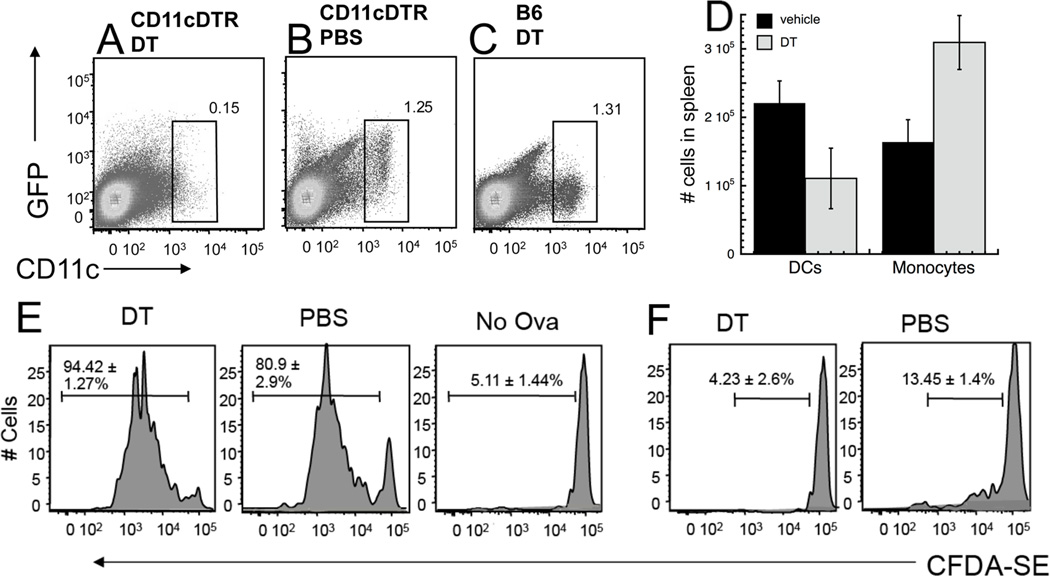
To determine if dendritic cells were specifically responsible for one or more phases of antigen persistence, we used CD11cDTR/GFP mice (22), in which DC express GFP and can be depleted by injecting the mice with diphtheria toxin (DT) (Fig. 5A–D). Although other studies have indicated that DT treatment of these mice can deplete other cell types (31, 32) we found that CD11c+ DC, but not F4/80+ monocyte/macrophages were depleted in VACV-infected mice treated with DT (Fig. 5D and manuscript in preparation). DT was administered once and antigen presentation assayed within 4d of depletion, a time point at which functional antigen presentation is not restored in our hands (not shown). In CD11cDTR/GFP mice infected with rVACV-OVA-FL, if DT was given during the phase of direct presentation by virally infected pAPCs, no difference in TCD8+ proliferation was seen between DT-injected and vehicle-injected mice (Fig. 5E). However, if DT was given during the later phase of cross-presentation by uninfected pAPCs (day 36 post-infection), TCD8+ proliferation was reduced to background levels in the DT-injected mice (Fig. 5F). We also used clodronate liposomes to deplete macrophages on d20 post infection, a procedure that leaves mature DCs intact (Fig. 6B). We did not detect any reduction in TCD8+ proliferation between mice treated with clodronate liposomes compared to those treated with PBS (Fig. 6A). This indicates that though DCs are responsible for cross-presentation by uninfected cells, neither macrophages nor DCs are solely responsible for persistent antigen presentation by infected cells.
Figure 5. Depleting CD11c+ cells in CD11cDTR/GFP mice prevents antigen presentation persistence, only during the later stage that depends on cross presentation.
CD11cDTR/GFP mice were treated with 4ng/g of DT (A, D) or PBS (B, D). C57BL/6 mice were treated with 4ng/g DT (C). Spleens were removed and CD11c+, GFP+ cells were analyzed by flow cytometry one day later (A–C). (D) - CD11cDTR/GFP mice were infected with 106 pfu rVACV. 15 days post-infection, VACV-infected mice were injected with DT (as above) or PBS (i.p.). Spleens were isolated 24 hours later, and single-cell suspensions of splenocytes were stained for monocyte/macrophages (F4/80+, CD11c−) and dendritic cells (CD11c+ F4/80−). Data represent the mean and SEM of at least 4 individual mice. (E, F) - CD11cDTR/GFP mice were infected with rVACV expressing OVA FL (E, F). 16 days (E) or 38 days (F) post infection mice were treated with 4ng/g DT. One day after DT treatment, OT-1.SJL T cells were labeled with CFDA-SE and transferred i.v. into previously infected mice. 3 days after transfer, spleens were removed and analyzed for CFDA-SE dilution. Representative histograms display gated CD45.1+ TCD8+ from individual mice of replicate experiments using 4 mice per condition. Gates represent the percentage of CD45.1+ TCD8+ cells that proliferated. Numbers represent the standard error of percent CD45.1+ TCD8+ proliferation.
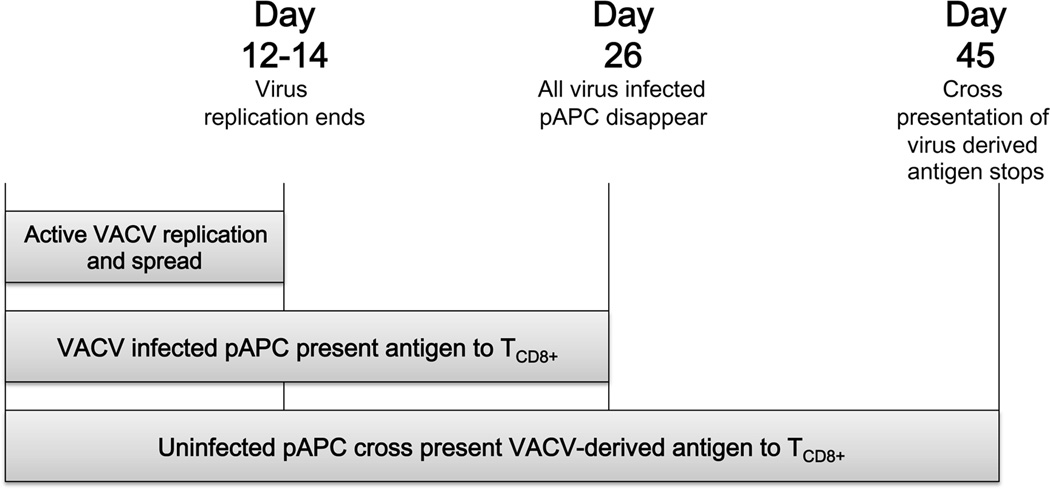
Here, we have determined that the persistence of antigen presentation consists of three distinct phases (Fig. 7). The first phase, viral replication, accounts for the first 14 days when ongoing virus replication can be detected. Both direct and cross presentation occur during this phase. The second phase, persistence of virally infected cells, is the next 14 days of prolonged antigen presentation. During this phase, virally infected cells persist with concomitant direct and cross presentation. The last phase, cross presentation, accounts for the final 18 days of antigen presentation when only cross presentation of antigen occurs.
Figure 7. Schematic of mechanisms responsible for prolonged antigen presentation following acute VACV infection.
Discussion
A robust TCD8+ response is vital in the control of viral infections. Prolonged antigen presentation is implicated in the generation and maintenance of memory TCD8+ (8, 33) and may be required for preferential localization of memory TCD8+ to secondary lymphoid organs draining a previous site of infection (34). Numerous publications have shown that prolonged antigen presentation occurs following an acute viral infection (6–8), although not following a bacterial infection (5). However, the mechanism responsible for antigen presentation persistence following viral clearance was unknown until now. Here, we utilized rVACV expressing different forms of the OVA protein to study the mechanism of prolonged antigen presentation. We determined that the persistence of antigen presentation consists of three distinct mechanistic phases: ongoing viral replication, persistence of virally infected cells, and cross presentation of antigen.
In agreement with data following acute infection with RNA viruses, we observed prolonged antigen presentation following an acute VACV infection. When mice were infected with rVACV-OVA FL, antigen presentation was observed for over 40 days (Fig. 1B). The prolonged antigen presentation was weeks longer than we were able to detect replicating virus, viral DNA or transcription of antigenic genes in mice (12–14 days, Fig. 1A). During this first phase of antigen presentation persistence, virus replication extended the amount of time antigen presentation was detected by 12–14 days. However, virus replication was not required for the persistence of antigen presentation, as mice infected with replication deficient viruses also displayed prolonged antigen presentation (Figs. 2 and 3A).
During the second phase of antigen presentation persistence, both direct and cross presentation can occur. To address the role of only direct presentation in the persistence of antigen presentation, we utilized a non-replicating rVACV-OVA MG with a half-life in the order of seconds (13). Due to the short half-life, only the originally infected cells are able to directly present antigen because OVA MG is likely degraded before it can be cross-presented (13). TCD8+ proliferation was observed for approximately 2 weeks in mice infected with rVACV expressing OVA MG (Fig. 3A). The number of peptide complexes produced from the OVA MG construct, even from a UV treated virus that does not replicate, is significantly greater than from a VACV expressing full length OVA (25), indicating that reduced production of peptide does not account for a reduced persistence of presentation. In addition, there is no evidence that peptide produced from a MG or full length construct has a different half life. Therefore, this result indicated that an originally infected cell was able to persist and directly present antigen for up to 2 weeks. The ability of a persistently infected cell to resist virus-induced lysis and present antigen is intriguing because VACV is described to cause death of infected APC, both in vitro and in vivo (28, 35).
DC were originally thought to be non-dividing cells with a reported lifespan of 2–3 days (36–38). If VACV is not causing DC death, the ability of DC to survive for 2 weeks is particularly interesting due to their reported short lifespan. However, more recent data suggests that DC, particularly conventional DC precursors, persist for up to 10 days (39, 40). In addition, daughter DC may present antigens captured from their progenitors, possibly prolonging the duration of antigen presentation (39, 41). It is possible that replicating DC extend the duration of antigen presentation observed in our system. OVA MG has a short half-life, requiring continuous peptide generation or stabilization of the peptide. It is possible that DC retain low levels of viral RNA that we cannot detect ex vivo, allowing continued low-level synthesis of the peptide by infected cells. Genomic RNA is detectable for two months following infection with VSV (42) and may contribute to prolonged antigen presentation following acute infection with VSV (8). However, VACV is a DNA virus that can replicate in the cytosol with little or no contribution from the host cell nucleus whereas VSV is an RNA virus that requires viral RNA to be transcribed in the nucleus during replication. Therefore, the difference in viral life cycle between VSV and VACV make the contribution of integrated nucleic acids to prolonged antigen presentation by VACV unlikely. Another possibility is the stabilization of OVA MG peptide by chaperones or MHC Class I molecules. However, this stabilization would have to occur for days and perhaps even weeks to account for the prolonged antigen presentation we observe by infected cells. The half-life of OVA257–264-Kb complexes is in the order of 360 minutes (43), making persistence for days unlikely. Even if peptide-MHC complexes are transferred to uninfected cells, a process known as cross-dressing (44) that is important in the expansion of memory TCD8+ following LCMV or VSV infection (45), the short half-life of these complexes may preclude a meaningful contribution to prolonged antigen presentation. Some peptides may bind to cytosolic chaperones (18) allowing them to survive for periods of time sufficient for them to be transferred to other cells for cross presentation. However, peptides that bind to chaperones under physiological conditions are the exception, not the rule, and this binding has not been demonstrated with the OVA257–264 peptide used in this study (46, 47).
When we depleted CD11c+ DC or macrophages, both of which are infected by VACV in vivo (28) using multiple methodologies, we did not observe a repeatable effect upon antigen presentation if depletion occurred prior to d20 post infection (Figs 5 and 6). However, our attempts to deplete both DC and macrophages simultaneously lead to death of the treated mice, so it is possible that both DC and macrophages can present persisting antigen, or that other infected immune cell types or somatic cells may contribute to the persistence of antigen presentation. We showed that the final 18 days of antigen presentation produced much lower levels of proliferation of antigen-specific TCD8+ made possible by cross presentation of antigen. Immunization of mice with infected presentation-incompetent β2M−/− cells produced the same 18 d period of antigen presentation that was lost when short-lived antigen that was not a substrate for cross presentation was expressed by rVACV. Depletion of CD11c+ cells during the final 18 d of presentation ablated the ability to trigger proliferation of OT1, likely indicating a requirement for cross presentation by specialized subsets of DC (Fig. 5E).
It has been proposed that DC have a unique ability to control the pH of the phagosome, with a pH of 7 and above that preserves antigen from rapid degradation and is optimal for cross presentation (48). gp91phox−/− mice that lack the gp91 subunit of NOX2, an NADPH oxidase that controls phagosomal pH, are deficient in cross presentation of antigen targeted to the CD205 receptor (48). However, we did not detect a significant difference in cross presentation of antigen between gp91phox−/− mice and C57BL/6 mice when virus or virus-infected cells were present (Fig. S4). This may be because the presence of virus induces inflammation that overcomes the requirement for modulation of phagosomal pH in DC (49) or because the requirement for gp91 in cross presentation is dependent upon targeting to the CD205 receptor. Antigen may be retained long-term in recently discovered antigen storage compartments found in mature DC (14). These antigen compartments are separate from early endosomal loading compartments and allow for a continuous supply of MHC Class I ligands for up to two weeks (14). It’s possible that these same storage compartments allow for the retention of antigen in DC following viral clearance, providing an antigen source for cross presentation. Alternatively, antigen may be retained long term in the form of immune complexes, which are stored long term for the production of antibody on the surface of follicular dendritic cells (50). Immune complexes are a substrate for cross presentation (51) and DC have been shown to cross present long-lived antibody-antigen complexes during persistent antigen presentation during an influenza virus infection (52, 53). It is possible that intact antigen within these complexes can be processed to maintain MHC Class I-restricted presentation during the cross presentation phase following VACV infection.
Many memory TCD8+ responses are studied 30 days post infection; however, we show here that original antigen presentation is still occurring at this time point following infection with rVACV. Antigen presentation after pathogen clearance may be capable of generating memory TCD8+ cells (8). Therefore, the memory response observed 30 days post infection may not be an accurate representation of the memory T cell population. It has previously been proposed that prolonged antigen presentation can trigger proliferating TCD8+ are subsequently deleted or enter an unresponsive state during the induction of peripheral tolerance (54). However, we found that VACV-specific TCD8+ triggered by persisting antigen presentation did become functionally active, albeit at a lower level that those triggered during the initial infection. Recently persistent antigen presentation following an acute RNA virus infection has been implicated in programming of memory TCD8+ {Leon, 2014 #36903;. The study of the phenotype of effector and memory TCD8+ produced following persistent antigen presentation after infection with the DNA virus VACV is beyond the scope of this study. However, the data yielded here will allow manipulation of the form of antigen contained within viral vectors or other vaccine preparations to allow the presentation of antigen for different periods of time, allowing the most effective and protective TCD8+ response to be generated following vaccination
Supplementary Material
Acknowledgments
We would like to thank Irene Reider and Melanie Epler for their excellent technical support. We acknowledge the contribution of Nate Sheaffer and Joe Bednarzyck of the Cell Science/Flow Cytometry Core Facility of the Section of Research Resources, Penn State College of Medicine.
This work was supported by NIH grants AI 056094 and AI070537 to CCN, U19 AI083008 (P.I. Luis Sigal), and training grant 5 T32 CA60395-15 (P.I. Aron Lukacher).
Abbreviations used in this article
- β2M
Beta-2 microglobulin
- CFDA-SE
5-(and 6-) carboxyfluorescein diacetate, succinimidyl ester
- DC
dendritic cell
- DT
diphtheria toxin
- FL
full-length
- LCMV
lymphocytic choriomeningitis virus
- LSM
lymphocyte separation medium
- MG
mini-gene
- pAPC
professional antigen-presenting cell
- rVACV
recombinant vaccinia virus
- VSV
vesicular stomatitis virus
References
- 1.Blair DA, Turner DL, Bose TO, Pham QM, Bouchard KR, Williams KJ, McAleer JP, Cauley LS, Vella AT, Lefrancois L. Duration of antigen availability influences the expansion and memory differentiation of T cells. Journal of immunology. 2011;187:2310–2321. doi: 10.4049/jimmunol.1100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belz GT, Wilson NS, Smith CM, Mount AM, Carbone FR, Heath WR. Bone marrow-derived cells expand memory CD8+ T cells in response to viral infections of the lung and skin. Eur J Immunol. 2006;36:327–335. doi: 10.1002/eji.200535432. [DOI] [PubMed] [Google Scholar]
- 3.Hermans IF, Ritchie DS, Yang J, Roberts JM, Ronchese F. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J Immunol. 2000;164:3095–3101. doi: 10.4049/jimmunol.164.6.3095. [DOI] [PubMed] [Google Scholar]
- 4.Hafalla JC, Sano G, Carvalho LH, Morrot A, Zavala F. Short-term antigen presentation and single clonal burst limit the magnitude of the CD8(+) T cell responses to malaria liver stages. Proc Natl Acad Sci U S A. 2002;99:11819–11824. doi: 10.1073/pnas.182189999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong P, Pamer EG. Feedback regulation of pathogen-specific T cell priming. Immunity. 2003;18:499–511. doi: 10.1016/s1074-7613(03)00081-5. [DOI] [PubMed] [Google Scholar]
- 6.Zammit DJ, Turner DL, Klonowski KD, Lefrancois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–449. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner DL, Cauley LS, Khanna KM, Lefrancois L. Persistent antigen presentation after acute vesicular stomatitis virus infection. J Virol. 2007;81:2039–2046. doi: 10.1128/JVI.02167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 10.Reits EA, Vos JC, Gromme M, Neefjes J. The major substrates for TAP in vivo are derived from newly synthesized proteins. Nature. 2000;404:774–778. doi: 10.1038/35008103. [DOI] [PubMed] [Google Scholar]
- 11.Donohue KB, Grant JM, Tewalt EF, Palmer DC, Theoret MR, Restifo NP, Norbury CC. Cross-priming utilizes antigen not available to the direct presentation pathway. Immunology. 2006;119:63–73. doi: 10.1111/j.1365-2567.2006.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serna A, Ramirez MC, Soukhanova A, Sigal LJ. Cutting edge: efficient MHC class I cross-presentation during early vaccinia infection requires the transfer of proteasomal intermediates between antigen donor and presenting cells. J Immunol. 2003;171:5668–5672. doi: 10.4049/jimmunol.171.11.5668. [DOI] [PubMed] [Google Scholar]
- 13.Norbury CC, Basta S, Donohue KB, Tscharke DC, Princiotta MF, Berglund P, Gibbs J, Bennink JR, Yewdell JW. CD8+ T cell cross-priming via transfer of proteasome substrates. Science. 2004;304:1318–1321. doi: 10.1126/science.1096378. [DOI] [PubMed] [Google Scholar]
- 14.van Montfort T, Thomas AA, Pollakis G, Paxton WA. Dendritic cells preferentially transfer CXCR4-using human immunodeficiency virus type 1 variants to CD4+ T lymphocytes in trans. Journal of virology. 2008;82:7886–7896. doi: 10.1128/JVI.00245-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma X, Serna A, Xu RH, Sigal LJ. The amino acid sequences flanking an antigenic determinant can strongly affect MHC class I cross-presentation without altering direct presentation. J Immunol. 2009;182:4601–4607. doi: 10.4049/jimmunol.0803806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu RH, Remakus S, Ma X, Roscoe F, Sigal LJ. Direct presentation is sufficient for an efficient anti-viral CD8+ T cell response. PLoS pathogens. 2010;6:e1000768. doi: 10.1371/journal.ppat.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reits E, Griekspoor A, Neijssen J, Groothuis T, Jalink K, van Veelen P, Janssen H, Calafat J, Drijfhout JW, Neefjes J. Peptide Diffusion, Protection, and Degradation in Nuclear and Cytoplasmic Compartments before Antigen Presentation by MHC Class I. Immunity. 2003;18:97–108. doi: 10.1016/s1074-7613(02)00511-3. [DOI] [PubMed] [Google Scholar]
- 18.Lev A, Takeda K, Zanker D, Maynard JC, Dimberu P, Waffarn E, Gibbs J, Netzer N, Princiotta MF, Neckers L, Picard D, Nicchitta CV, Chen W, Reiter Y, Bennink JR, Yewdell JW. The exception that reinforces the rule: crosspriming by cytosolic peptides that escape degradation. Immunity. 2008;28:787–798. doi: 10.1016/j.immuni.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 20.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 21.Burnett SH, Kershen EJ, Zhang J, Zeng L, Straley SC, Kaplan AM, Cohen DA. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol. 2004;75:612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- 22.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer E, Littman D, Lang R. In Vivo Depletion of CD11c(+) Dendritic Cells Abrogates Priming of CD8(+) T Cells by Exogenous Cell-Associated Antigens. Immunity. 2002;17:211. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klochkov DB, Gavrilov AA, Vassetzky YS, Razin SV. Early replication timing of the chicken alpha-globin gene domain correlates with its open chromatin state in cells of different lineages. Genomics. 2009;93:481–486. doi: 10.1016/j.ygeno.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes DM, Vidard L, Rock KL. Characterization of MHC class II-presented peptides generated from an antigen targeted to different endocytic compartments. Eur J Immunol. 2000;30:2333–2343. doi: 10.1002/1521-4141(2000)30:8<2333::AID-IMMU2333>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 25.Fischer MA, Tscharke DC, Donohue KB, Truckenmiller ME, Norbury CC. Reduction of vector gene expression increases foreign antigen-specific CD8+ T-cell priming. J Gen Virol. 2007;88:2378–2386. doi: 10.1099/vir.0.83107-0. [DOI] [PubMed] [Google Scholar]
- 26.Wilson NS, Behrens GM, Lundie RJ, Smith CM, Waithman J, Young L, Forehan SP, Mount A, Steptoe RJ, Shortman KD, de Koning-Ward TF, Belz GT, Carbone FR, Crabb BS, Heath WR, Villadangos JA. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat Immunol. 2006;7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- 27.Daeron M, Neauport-Sautes C, Blank U, Fridman WH. 2.4G2, a monoclonal antibody to macrophage Fc gamma receptors, reacts with murine T cell Fc gamma receptors and IgG-binding factors. Eur J Immunol. 1986;16:1545–1550. doi: 10.1002/eji.1830161213. [DOI] [PubMed] [Google Scholar]
- 28.Norbury CC, Malide D, Gibbs JS, Bennink JR, Yewdell JW. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat Immunol. 2002;3:265–271. doi: 10.1038/ni762. [DOI] [PubMed] [Google Scholar]
- 29.Schell AM, Granger EL, Koczot F, Fischer MA, Norbury CC. Dendritic cell migration limits the duration of CD8+ T-cell priming to peripheral viral antigen. Journal of virology. 2010;84:3586–3594. doi: 10.1128/JVI.01975-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsung K, Yim JH, Marti W, Buller RM, Norton JA. Gene expression and cytopathic effect of vaccinia virus inactivated by psoralen and long-wave UV light. J Virol. 1996;70:165–171. doi: 10.1128/jvi.70.1.165-171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Probst HC, Tschannen K, Odermatt B, Schwendener R, Zinkernagel RM, Van Den Broek M. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin Exp Immunol. 2005;141:398–404. doi: 10.1111/j.1365-2249.2005.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, Idoyaga J, Cheong C, Yao KH, Niec RE, Nussenzweig MC. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. The Journal of experimental medicine. 2012;209:1153–1165. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cockburn IA, Chen YC, Overstreet MG, Lees JR, van Rooijen N, Farber DL, Zavala F. Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites. PLoS pathogens. 2010;6:e1000877. doi: 10.1371/journal.ppat.1000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim TS, Hufford MM, Sun J, Fu YX, Braciale TJ. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. The Journal of experimental medicine. 2010;207:1161–1172. doi: 10.1084/jem.20092017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engelmayer J, Larsson M, Subklewe M, Chahroudi A, Cox WI, Steinman RM, Bhardwaj N. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J Immunol. 1999;163:6762–6768. [PubMed] [Google Scholar]
- 36.Ruedl C, Koebel P, Bachmann M, Hess M, Karjalainen K. Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes. Journal of immunology. 2000;165:4910–4916. doi: 10.4049/jimmunol.165.9.4910. [DOI] [PubMed] [Google Scholar]
- 37.Kamath AT, Pooley J, O'Keeffe MA, Vremec D, Zhan Y, Lew AM, D'Amico A, Wu L, Tough DF, Shortman K. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J Immunol. 2000;165:6762–6770. doi: 10.4049/jimmunol.165.12.6762. [DOI] [PubMed] [Google Scholar]
- 38.Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100:1734–1741. [PubMed] [Google Scholar]
- 39.Diao J, Winter E, Chen W, Xu F, Cattral MS. Antigen transmission by replicating antigen-bearing dendritic cells. J Immunol. 2007;179:2713–2721. doi: 10.4049/jimmunol.179.5.2713. [DOI] [PubMed] [Google Scholar]
- 40.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O'Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 41.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nature immunology. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 42.Simon ID, Publicover J, Rose JK. Replication and propagation of attenuated vesicular stomatitis virus vectors in vivo: vector spread correlates with induction of immune responses and persistence of genomic RNA. J Virol. 2007;81:2078–2082. doi: 10.1128/JVI.02525-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howarth M, Williams A, Tolstrup AB, Elliott T. Tapasin enhances MHC class I peptide presentation according to peptide half-life. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11737–11742. doi: 10.1073/pnas.0306294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dolan BP, Gibbs KD, Jr, Ostrand-Rosenberg S. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J Immunol. 2006;177:6018–6024. doi: 10.4049/jimmunol.177.9.6018. [DOI] [PubMed] [Google Scholar]
- 45.Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471:629–632. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker-LePain JC, Reed RC, Nicchitta CV. ISO: a critical evaluation of the role of peptides in heat shock/chaperone protein-mediated tumor rejection. Curr Opin Immunol. 2003;15:89–94. doi: 10.1016/s0952791502000067. [DOI] [PubMed] [Google Scholar]
- 47.Nicchitta CV. Role of chaperones in antigen processing. Immunol Invest. 2000;29:101–104. doi: 10.3109/08820130009062290. [DOI] [PubMed] [Google Scholar]
- 48.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Dumenil AM, Seabra MC, Raposo G, Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 49.Segura E, Albiston AL, Wicks IP, Chai SY, Villadangos JA. Different cross-presentation pathways in steady-state and inflammatory dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20377–20381. doi: 10.1073/pnas.0910295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bachmann MF, Odermatt B, Hengartner H, Zinkernagel RM. Induction of long-lived germinal centers associated with persisting antigen after viral infection. J Exp Med. 1996;183:2259–2269. doi: 10.1084/jem.183.5.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leon B, Ballesteros-Tato A, Randall TD, Lund FE. Prolonged antigen presentation by immune complex-binding dendritic cells programs the proliferative capacity of memory CD8 T cells. J Exp Med. 2014 doi: 10.1084/jem.20131692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ballesteros-Tato A, Leon B, Lee BO, Lund FE, Randall TD. Epitope-specific regulation of memory programming by differential duration of antigen presentation to influenza-specific CD8(+) T cells. Immunity. 2014;41:127–140. doi: 10.1016/j.immuni.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernandez J, Aung S, Marquardt K, Sherman LA. Uncoupling of proliferative potential and gain of effector function by CD8(+) T cells responding to self-antigens. J Exp Med. 2002;196:323–333. doi: 10.1084/jem.20011612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.