Abstract
In-hospital biliary complications (BCs) after liver transplantation (LT) are reported in up to 20% of patients and contribute to poor outcomes and increased costs. Existing single center outcome and cost analyses studies are limited in scope. This is a cross-sectional analysis of national data involving 7,967 patients transplanted between 2011–12 with the primary aim of determining the association between BCs and clinical outcomes and costs. Age, race, diagnosis, and severity of illness are associated with the development of BCs. BCs develop in 14.6% of LT recipients and have substantial implications for peri-operative outcomes, including length of hospital and ICU stay (27·9 vs 19·6 mean days, p<0·001 and 12·0 vs 8·3 mean days, p<0·001 respectively), in-hospital morbidity (39% vs 27%, p<0·001), 30-day readmissions (14·8% vs 11·2%, p<0·001), and in-hospital mortality (5·8% vs 4·0%, p<0·001). BCs contributed to a mean increase in in-hospital costs of $36,212 (p<0·001), due to increases in accommodations ($9,539, p<0·001), surgical services ($3,988, p<0·001), and pharmacy services ($8,445, p<0·001). BCs are a predominant etiology for in-hospital morbidity and mortality, while contributing significantly to the high cost of LT. Efforts should be focused on understanding salient and modifiable risk factors, while developing innovative strategies to reduce BCs.
Keywords: Biliary complications, liver transplantation, clinical outcomes, costs
Introduction
The etiology of biliary complications (BCs) after liver transplantation (LT) are multifactorial and are due, in part, to a vulnerable bile duct blood supply and are a major source of morbidity. They have been reported to occur in up to 20% of LT recipients(1–5) and ischemic-type biliary lesions are the third most common reason for retransplantation(1, 2). Risk factors include donor age, prolonged ischemia time, use of marginal grafts, donation after cardiac death (DCD) donors, macrovesicular steatosis, hepatic artery thrombosis, ABO blood type incompatibility, cytomegalovirus infection, recurrence of primary disease, partial LTs, Child-Pugh score, and the method of biliary reconstruction.(6–11)
LT is the definitive therapy for end-stage liver diseases and acute liver failure. LT is also one of the most expensive and complex medical procedures available worldwide.(12) Due to the increasing costs, significant pressure has been applied by both private and public health care payers to justify the continued widespread availability of LT.(13) The most effective way to provide such justification is to show that the cost-outcome relationship in LT is being optimized. In the current financial and regulatory climate, it is imperative that clinical outcomes after LT improve whilst costs associated with the procedure decrease. BCs have been demonstrated to cause prolonged hospital stays, long-term complications, inferior quality of life, increased costs, and exacerbation of the donor organ shortage.(4, 14) Although much is known about the risks and outcomes associated with BCs, there is a paucity of large-scale studies analyzing the costs, resource utilization, and clinical outcomes of LT recipients that develop these complications. Understanding these clinical and economic implications are important to provide justification for structured programs to minimize the incidence and severity of this common complication. The objective of this study is to compare the in-hospital clinical outcomes and costs of LT recipients that developed BCs versus those without such complications, with projection analyses to determine the impact of reducing the incidence of such complications.
Methods
Study Design
This analysis of United States (US) national data was approved by the institutional review board (IRB) in order to determine the association between BCs and outcomes in LT. A database was developed by combining various components of 2011 to 2012 aggregate variables downloaded and integrated from several datasets contained within one primary data source (www.uhc.edu). The University HealthSystem Consortium (UHC) is an alliance of 416 academic medical centers and affiliates in the United States which contains detailed peri-operative clinical data that is gathered, aggregated at the hospital level and available for download by affiliate members. Specific information available from UHC includes mean and variance data on patient sociodemographics, acuity of illness, admission diagnoses and procedures, hospital and intensive care unit (ICU) lengths of stay, in-hospital complications, mortality, readmissions and direct and total costs.
Patients
All adult LT recipients (>18 years of age) who received a transplant within a UHC-affiliated center in 2011 and 2012 were included. Seventy out of 130 (54%) transplant programs that performed adult LTs during 2011–12 were documented as members of UHC. These centers performed nearly two-thirds of all adult LTs during this timeframe (7,967 of 12,631, 63%). Patients that received transplants from non-UHC affiliated centers were excluded from this analysis.
Study Objectives
The primary aim of this study was to determine the association between BCs and peri-operative clinical outcomes and costs. The outcomes of interest included observed and expected hospital length of stay (LOS), ICU LOS, in-hospital non-biliary complications, in-hospital mortality (observed and expected), blood transfusions, and readmissions. Cost outcomes analyses included the mean difference in overall costs, direct costs, and service specific costs between patients with and without BCs. Service specific costs were delineated into service groups, which included accommodations (daily costs for room), ancillary services, cardiac diagnostic services, other diagnostic imaging (ultrasound, X-ray, radiology, percutaneous transhepatic cholangiography, endoscopic retrograde cholangiopancreatography, etc), laboratory/pathology/blood bank (including biopsy and transfusion costs), other diagnostic services, surgical services (operating room costs), organ procurement services (pass through costs from the organ procurement organization and Medicare Cost Report), pharmacy services (medications) and miscellaneous.
Study Definitions
Peri-operative quality measure definitions included mean LOS and ICU LOS, defined as mean days in the hospital and ICU following the transplant surgical event. In-hospital complications were a composite definition of the mean percentage of in-hospital complications occurring in patients undergoing LT. These complications were determined through international statistical classification of diseases and related health problems (ICD-9) coding and included the following: reopening the surgical site, pneumonia, sepsis, UTI, stroke, acute myocardial infarction, shock, gastrointestinal (GI) hemorrhage, and wound infection. BCs were determined by using diagnostic coding and included the following ICD-9 codes: 576.1, .2, .3, .4, .8 & .9 (cholangitis, obstruction, perforation, fistula, biliary tract disorder not elsewhere classified (NEC) and biliary tract disorder not otherwise specified (NOS)). Readmissions were defined as the percent of LT recipients readmitted to the index hospital within seven, 14 and 30-days following discharge. All events, other than readmissions, were only included if they occurred during the hospitalization. Due to limitations of UHC data reporting, post-discharge events (other than readmissions) were not captured within this analysis. Expected LOS, cost, and mortality were also calculated and compared across groups. These risk-adjustment equations were derived from binary logistic regression analyses conducted by UHC on patient-level data and reported as aggregate data for each transplant center. UHC utilizes the diagnosis specific APR-DRG based analyses developed by 3M Health Information Systems (Murray, UT). This system is employed to determine severity of illness at admission and utilized to estimate expected LOS, expected costs, and expected mortality. These expected data are compared to observed data to determine indexes for each of these three outcomes.
Costs were determined using a standardized cost definition used by UHC to uniformly compare hospital reported costs across a similar platform. UHC calculates costs by gathering the total charges for each inpatient discharge based on administrative claims submissions. This data is then divided into line item charges grouped by revenue codes and multiplying this value by the ratio of costs to charges (RCCs) that are reported by each hospital through their Medicare Cost Report. These costs are then adjusted by an area wage index applied to the labor portion of cost, which allows comparison of hospitals across various geographic areas of the U.S. Direct costs are calculated from adjusted RCCs by removing costs associated with departments classified as overhead (environmental services, utilities, etc).
Statistical Analysis Plan
For statistical comparisons, patients that developed in-hospital BCs following LT were grouped and aggregate data was compared with control group aggregate data, defined as patients undergoing LT and not developing inhospital BCs. Comparisons were made between these two cohorts for baseline sociodemographics, peri-operative clinical outcomes, and in-hospital direct, total, and categorical cost differences. Using projection analysis, the impact of reducing BCs by 20% was estimated for both clinical and cost outcomes. These projections assumed BCs rates were similar across between UHC and non-UHC transplant centers. For categorical data, statistical comparisons were made using the Pearson’s chi square test, with continuous variables being compared using the Student’s T-test for normally distributed data and the Mann-Whitney U-test for non-parametric data. Normal distribution was determined using Levene’s Test for Equality of Variances. Data was exported from UHC into MS Excel 2010 (Microsoft Corp, Seattle, WA) and statistical analyses were conducted using SPSS version 20.0 (IBM Corp, Armonk, NY).
Results
Baseline patient characteristics
A total of 7,967 adult LT recipients from calendar years 2011 and 2012 were included in this analysis. These LTs occurred within 70 transplant programs, all members of UHC. This cohort represents 54% of all adult LT programs within the US and 63% of all adult LTs that were performed during this two-year timeframe (7,967 of 12,631). Of these 7,967 LTs, 1,160 (14·6%) developed in-hospital BCs per diagnostic coding. The types of BCs included cholangitis (47%), obstruction (23%), NEC (29%), perforation (0.2%), fistula (0.2%) and NOS (0.2%). Baseline characteristic comparisons between the BCs and control cohorts are displayed in Table 1. Patients that developed a post-LT BCs tended to be younger in age (p<0.001), and African-Americans were at a 27% higher risk of developing BCs (p=0.01). Additional baseline differences between cohorts included primary diagnosis, with BC patients more likely to carry a diagnosis of fulminant hepatic failure, autoimmune hepatitis, or biliary cirrhosis. Patients that were assessed to have major or extreme severity of illness, based on APR-DRG indexing, were 19% more likely to develop a post-LT BCs (p<0·001). Gender, type of primary insurance, and comorbidities did not appear to influence the incidence of BCs.
Table 1.
Baseline Characteristics within Each Cohort
| Characteristics | Biliary Complications (n=1,160) | Without Biliary Complications (n=6,807) | p-Value | |
|---|---|---|---|---|
| Age - n (%) | 18 – 30 years | 115 (10) | 200 (3) | <0.001 |
| 31 – 40 years | 116 (10) | 276 (4) | <0.001 | |
| 41 – 50 years | 232 (20) | 1,078 (16) | <0.001 | |
| 51 – 60 years | 410 (35) | 3,154 (46) | <0.001 | |
| 61 – 70 years | 277 (24) | 1,971 (29) | <0.001 | |
| >70 years | 10 (1) | 128 (2) | 0.011 | |
| Gender - n (%) | Male | 772 (67) | 4,598 (68) | 0.52 |
| Female | 388 (33) | 2,209 (32) | ||
| Race - n (%) | African-American | 133 (11) | 615 (9) | 0.01 |
| Caucasian/Other | 1,027 (89) | 6,192 (91) | ||
| Diagnosis - n (%) | Hepatitis C | 298 (26) | 3241 (48) | <0.001 |
| Fulminant | 106 (9) | 382 (6) | <0.001 | |
| Hepatocellular Carcinoma | 177 (15) | 2321 (34) | <0.001 | |
| Alcohol | 187 (16) | 2130 (31) | <0.001 | |
| Autoimmune | 77 (7) | 285 (4) | <0.001 | |
| Biliary Cirrhosis | 91 (8) | 264 (4) | <0.001 | |
| Comorbidities - n (%) | Hepatorenal Syndrome | 226 (19) | 1341 (20) | 0.905 |
| End-Stage Renal Disease | 87 (8) | 591 (9) | 0.191 | |
| Ascites | 546 (47) | 3315 (49) | 0.309 | |
| Hepatopulmonary Syndrome | 14 (1) | 120 (2) | 0.216 | |
| Hepatic Encephalopathy | 254 (22) | 1431 (21) | 0.509 | |
| Primary Insurance - n (%) | Medicare | 291 (25) | 1,818 (27) | 0.264 |
| Medicaid | 137 (12) | 855 (13) | 0.501 | |
| Private | 701 (60) | 3,922 (58) | 0.077 | |
| Military | 12 (1) | 66 (1) | 0.872 | |
| Other | 19 (2) | 146 (2) | 0.315 | |
| Admission Severity of Illness - n (%) | Minor | 52 (4) | 456 (7) | 0.003 |
| Moderate | 278 (24) | 2,258 (33) | <0.001 | |
| Major | 456 (39) | 2,501 (37) | 0.100 | |
| Extreme | 374 (32) | 1,592 (23) | <0.001 | |
| Case Mix Index - n (%) | 9.87±2.08 | 9.15±1.39 | <0.001 |
Types of biliary complications: cholangitis 627 (47%), obstruction 312 (23%), NEC 384 (29%), perforation 2 (0.2%), fistula 3 (0.2%), NOS 3 (0.2%)
Peri-Operative Clinical Outcomes based on the Development of BCs
Development of in-hospital BCs had a substantial impact on the majority of measured peri-operative outcomes measured (Table 2). In-hospital mortality was 50% higher in patients with BCs 5·8% vs. 4%, p<0·001), while 30-day readmissions were 36% more frequent (15% vs. 11%, p<0·001). Finally, patients with post-LT BCs had a 29% higher mortality index as compared to the non-BC cohort (1·12 vs. 0·87, p<0·001). The observed mean hospital LOS was 8.3 days longer and the mean ICU LOS was 3·7 days longer in LT recipients that developed BCs (p<0·001). This translated into a 31% higher LOS index for the BCs cohort (P<0·001). Patients that developed BCs also had a 31% increased risk of developing in-hospital non-biliary complications, which was predominantly driven by reoperation and infections. Although blood product transfusion rates were similar, the BCs cohort received more total transfusions, as evidenced by the higher number of mean days a patient required transfusion services (6·7 days vs. 4·9 days, p<0·001).
Table 2.
Peri-Operative Clinical Outcomes based on the Development of a Biliary Complications
| Outcomes | Biliary Complications (n=1,160) | Without Biliary Complications (n=6,807) | p-Value | |
|---|---|---|---|---|
| Mean Length of Stay Observed - days ± SD | 27.9 ± 26.9 | 19.6 ± 20.9 | <0.001 | |
| Mean Length of Stay Expected - days ± SD | 21.5 ± 5.1 | 19.6 ± 3.9 | <0.001 | |
| Length of Stay Index - O/E | 1.28 ± 0.51 | 0.98 ± 0.35 | <0.001 | |
| ICU Admissions - % | 92% | 93% | 0.217 | |
| Mean ICU Length of Stay - days ± SD | 12.0 ± 8.4 | 8.3 ± 9.1 | <0.001 | |
| with Additional Complications* - % | 39% | 27% | <0.001 | |
| Transfused with Any Blood Product - % | 91% | 91% | 1.000 | |
| Days of Transfusions - days ± SD | 6.7 ± 3.3 | 4.9 ± 2.6 | <0.001 | |
| In-Hospital Mortality Observed - % | 5.8% | 4.0% | 0.007 | |
| In-Hospital Mortality Expected - % | 5.1% | 4.6% | 0.452 | |
| Mortality Index - O/E | 1.12 ± 1.67 | 0.87 ± 1.32 | <0.001 | |
| Readmissions - % | 7-Day | 8.0% | 5.3% | <0.001 |
| 14-Day | 11.6% | 8.0% | <0.001 | |
| 30-Day | 14.8% | 11.2% | <0.001 |
Additional Complications
Defined as in-hospital stroke, aspiration pneumonia, GI hemorrhage, catheter associated UTI, shock due to anesthesia, reopening of surgical site, mechanical complications due to device, acute MI, arrhythmias, coma, nosocomial pneumonia, wound infection, sepsis
In addition, patients that developed BCs were significantly more likely to have in-hospital endoscopic retrograde cholangiopancreatography (ERCP, 6% vs. 1%, p<0.001), cholangiography (16% vs. 9%, p<0.001) and percutaneous transhepatic cholangiography (PTC 5% vs 2%, p<0.001).
Costs associated with the development of BCs
Figure 1 displays the differences between the BCs and control cohort in the overall costs, observed direct costs, and expected direct costs. Patients that developed BCs had over $36,000 in increased overall in-hospital costs (mean difference $36,212, 95% CI $29,349 – 43,075, p<0·001). Differences in observed in-hospital direct costs (excluding hospital overhead) were also significant ($24,900, 95% CI $19,923 – 29,877, p<0·001), leading to an 11% increase in the direct cost index for patients that developed BCs (see right side of Figure 1, p<0·001).
Figure 1. Differences between the BCs and control cohort in the overall costs, observed direct costs, and expected direct costs.
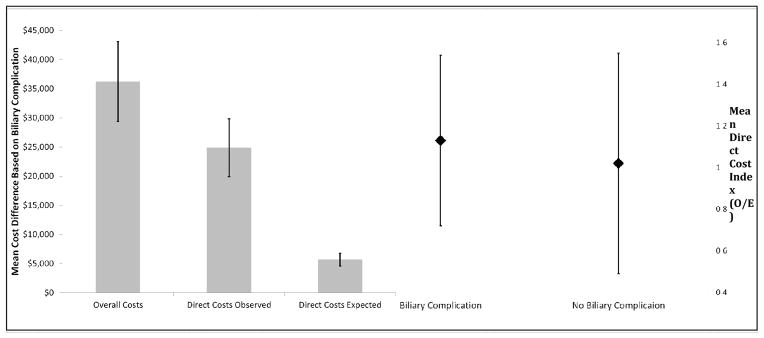
The bar graph to the left represents costs differences, comparing those that developed a biliary complication to those that did not. The higher number represents increased costs that were associated with biliary complications. For example, overall mean cost difference was $225,303 – $189,091. The chart to the right represents the mean direct cost index, calculating using the observed-to-expected cost ratios for those with and without biliary complications. A direct cost index of 1·00 represents an expected direct cost. The direct cost index for patients with biliary complications was 1·13±0·41 vs. 1·02±0·53 for those without such complication (p<0·001).
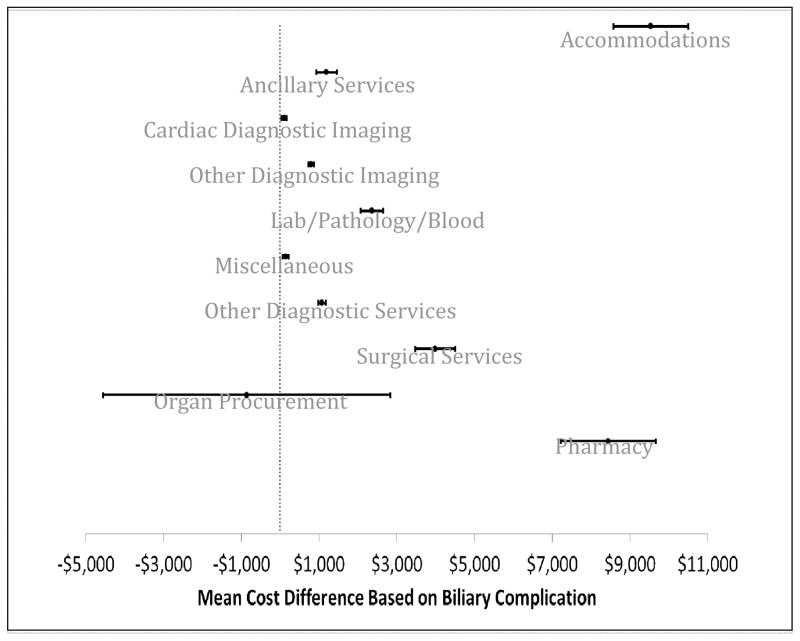
To improve the contributing factors associated with higher costs in patients with BCs, individual categories that make up the composite overall direct costs were analyzed separately in Figure 2. This data demonstrates overall a wide range of service groups, including accommodations, diagnostics, laboratories, operation costs and pharmacy services in BCs patients, drove higher costs. Cardiac diagnostic imaging, organ procurement, and miscellaneous costs were similar between the groups. The predominant costs differences included three service groups, accommodations (cost difference $9,539, 95% CI $9,580 – 10,497, p<0·001), surgical services (cost difference $3,988, 95% CI $3,478 – 4,498, p<0·001), and pharmacy services (cost difference $8,445, 95% CI $7,223 – 9,667, p<0·001).
Figure 2. Mean cost difference between cohorts (those that developed a perioperative biliary complication vs. those that did not).
The mean cost difference is delineated based on categories, with a positive cost representing an increased cost associated with a biliary complication and a negative costs representing a lower cost associated with biliary complications. The error bars represent the 95% confidence interval. Bars crossing the dotted line (0-axis) are statistically insignificant cost differences.
Projection Analysis
Cost analyses were based on the observed costs for the 1,160 patients that developed BCs. The incidence of BCs in this cohort of patients was 14·6%. Using projection analyses and applying similar rates across the US LT population, we estimate that BCs following LT cost the US health care system $33·3 million annually, primarily driven by increased LOS leading to $8·7 million in hospital room costs, $3·6 million in additional operative costs, and $7·7 million in pharmacy costs. Annually, BCs in US LT recipients accounted for over 7,641 additional hospital days, 3,406 of which are spent in the ICU, and approximately 21 additional in-hospital deaths per year.
Strategies to prevent or reduce the incidence of BCs have the potential to substantially influence clinical and economic outcomes in LT. A 20% reduction in the incidence of BCs across the US would result in a savings of over $6·7 million in overall costs to the health system, a reduction of over 1,528 hospital days, and potentially prevent more than 4 in-hospital deaths per year.
Discussion
Liver transplantation is a technically challenging procedure fraught with potential complications. Risk factors for these complications are often not modifiable in the pre-operative course. Despite refinements in surgical techniques, BCs continue to be a predominant cause of morbidity, graft loss, and mortality in LT recipients.(15) The results of this analysis demonstrate that, in the contemporary era of LT, BCs have a profound clinical and economic impact as evidenced by peri-operative outcomes, including in-hospital mortality, and health care costs.
This analysis demonstrated that the hospital and ICU LOS, risk of developing in-hospital non-biliary complications, total transfusions, in-hospital mortality, and 30-day readmissions are all substantially higher in LT recipients developing BCs. Patients with BCs are also more likely to have ERCP, cholangiography, and PTC. Likewise, patients developing BCs had significantly increased overall in-hospital costs, driven by a wide range of individual service groups, including accommodations, diagnostics, laboratories, operation costs, and pharmacy services.
No previous studies have analyzed costs associated with BCs at a national, or even multicenter level. A single center study by Englesbe et. al. from the University of Michigan Health System, which included 256 LT recipients, was the first to address the issue of costs associated with BCs. In this single center study the authors report that recipients that developed BCs had an average hospital cost of $214,968 vs. $149,897 for patients that did not develop BCs, a difference of $65,071 (p<0·001). Although the investigators make a strong financial case for transplant surgical quality improvement(16), the single center study did not find significant differences in known risk factors for BCs including DCD donors and preservation solution used. While the study reported significant differences in the number of hospital days during the first 6 months post-transplant, readmissions and clinic visits were not significantly affected by BCs.
An important finding of this study, that BCs significantly influence patient survival, is in contrast with other previous studies. These investigations did not find that BCs were significantly associated with decreased patient or graft survival. Qian et al studied LT in 230 patients performed over a period of 11 years at Queen Mary Hospital in Hong Kong and demonstrated that BCs had no significant impact on patient survival.(6) Kling et al evaluated 47 living related pediatric LT patients that developed BCs and reported that they were not associated with decreased patient survival.(17) A 348 patient study by Gunawansa et al at the National Hospital of Sri Lanka reported BCs in 20% of patients and demonstrated no significant associated mortality.(18) The analysis presented in this study indicates that patients that developed post-LT in-hospital BCs had a 29% higher mortality index that translated into 21 more deaths per year. It is likely that the failure of previous analyses to demonstrate a higher mortality rate in LT recipients that develop BCs may be due to small sample-sizes, low mortality rates and a lack of statistical power. This analysis, although only able to assess in-hospital events, did capture a large US national cohort, and thus is powered to detect smaller clinical differences.
In addition to the risk factors discussed in the introduction, other studies have shown that preoperative serum bilirubin level, use of stent or T-tube splinting of the anastomosis, and living-donor liver grafts are independent risk factors for BCs.(6, 19) As many risk factors cannot be modified, methods to counteract these risk factors and improvements in surgical techniques are important to implement to minimize the occurrence of BCs. For instance, Qian et. al. suggest that technical refinements in homeostasis and liberal infusion of fresh frozen plasma and platelets may reduce the complications associated with high serum bilirubin levels, while splinting of the choledochocholedochostomy by T tube or the hepaticojejunostomy by an internal stent are unnecessary.(6) In a retrospective study of 1,843 patients at Universitätsmedizin Berlin, Germany, Heidenhain et. al. observed that organs perfused with University of Wisconsin solution developed ischemic type biliary lesions significantly more frequently than those perfused with Histidine-Tryptophan-Ketoglutarate.(20) A few studies suggest using back-table pressure perfusion of the hepatic artery for efficient perfusion of the biliary tract capillary system to significantly reduce the risk of BCs.(20, 21)
Although a number of known risk factors are not modifiable, several are. For instance, in animal models of transplantation, minocycline, N-methyl-4-isoleucine cyclosporine, and Heme oxygenase-1 overexpression have demonstrated the ability to mitigate storage and reperfusion injury,(22, 23) thus reducing the severity of ischemic-type BCs. In a previous trial, we demonstrated the safe use of highly steatotic donor livers by utilizing a detailed donor/recipient matching algorithm.(24) This algorithm performed risk factor matching, such that highly marginal organs were only utilized in low risk recipients. Such a strategy applied to BCs risk factors, may potentially mitigate non-modifiable risk by reducing the incidence or severity of BCs as well. Several pharmacologic approaches are also being explored, including using beta- and alpha-adrenergic blockers, glutathione, and others to effectively reduce steatosis,(25) which may further reduce BCs risk. New therapies that treat CMV infection, such as CMX001(26) could also prove to be beneficial. Thus, novel methods to ameliorate risk factors are under development and should provide more avenues to effectively reduce the incidence and impact of post-LT BCs.
There are a number of limitations to this study worthy of discussion. Firstly, due to the limitations associated with the type of data variables available within this analysis, we were unable to analyze known risk factors associated with BCs, including donor characteristics, donor risk index, organ features, ischemia times and MELD scores. Although these limitations were inevitable, it should be noted that the primary aim of this study was not to analyze risk factors associated with BCs, which have been well described in previous studies, but to determine the clinical and economic outcomes associated with BCs, which the current literature is lacking. Additional limitations to this study include the fact that the analysis relied on ICD-9 code for billing data to determine outcomes and costs and this may have underestimated the number of BCs. Additionally, this analysis only included clinical events that occurred within the initial index hospitalization. It should be noted that the UHC database includes only 70 of the 130 transplant centers that performed LTs during the study time period; although, this does account for 63% of the total LT performed within the US in 2011 to 2012. The cost analysis may not be generalizable outside of the US, but is a reasonable reflection of current practices. Although BCs manifesting post-discharge are not captured in the present study, it is anticipated that it will likely increase the costs associated with BCs and reflects the importance of BC’s on overall costs.
Interpretation
This cross-sectional analysis of a national well-representative cohort of LT recipients clearly demonstrates that post-LT BCs to be a clinically and financially significant complication, leading to a high rate of morbidity and mortality while dramatically increasing costs and resources. Effective interventions aimed at reducing BCs will likely have a significant impact on patient outcomes and health care expenditures following LT. As the US and world populations grow increasingly obese, end stage liver disease necessitating LT is increasingly common. Continued broad implementation of LT is likely to be predicated on its efficacy and cost effectiveness. Our study provides robust evidence to support the development of innovative interventions aimed at minimizing the incidence and severity of BCs and to optimize care.
Acknowledgments
No extramural funding source associated with this study.
Abbreviations
- BCs
Biliary Complications
- LT
Liver Transplantation
- DCD
Donation After Cardiac Death
- UHC
University HealthSystem Consortium
- ICU
Intensive Care Unit
- LOS
Length of Stay
- GI
Gastrointestinal
- NEC
Not Elsewhere Classified
- NOS
Not Otherwise Specified
- ICD-9
International Statistical Classification of Diseases and Related Health Problems
- US
United States
Footnotes
Disclosure
No authors have any competing interests. An ethics statement was not required for this work.
References
- 1.Colonna JO, 2nd, Shaked A, Gomes AS, Colquhoun SD, Jurim O, McDiarmid SV, et al. Biliary strictures complicating liver transplantation. Incidence, pathogenesis, management, and outcome. Ann Surg. 1992;216(3):344–50. doi: 10.1097/00000658-199209000-00014. discussion 50–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenhain C, Heise M, Jonas S, Ben-Asseur M, Puhl G, Mittler J, et al. Retrograde reperfusion via vena cava lowers the risk of initial nonfunction but increases the risk of ischemic-type biliary lesions in liver transplantation--a randomized clinical trial. Transpl Int. 2006;19(9):738–48. doi: 10.1111/j.1432-2277.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 3.Neuhaus P, Blumhardt G, Bechstein WO, Steffen R, Platz KP, Keck H. Technique and results of biliary reconstruction using side-to-side choledochocholedochostomy in 300 orthotopic liver transplants. Ann Surg. 1994;219(4):426–34. doi: 10.1097/00000658-199404000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez-Urdazpal L, Gores GJ, Ward EM, Maus TP, Wahlstrom HE, Moore SB, et al. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology. 1992;16(1):49–53. doi: 10.1002/hep.1840160110. [DOI] [PubMed] [Google Scholar]
- 5.Adam R, Bismuth H, Diamond T, Ducot B, Morino M, Astarcioglu I, et al. Effect of extended cold ischaemia with UW solution on graft function after liver transplantation. Lancet. 1992;340(8832):1373–6. doi: 10.1016/0140-6736(92)92559-x. [DOI] [PubMed] [Google Scholar]
- 6.Qian YB, Liu CL, Lo CM, Fan ST. Risk factors for biliary complications after liver transplantation. Arch Surg. 2004;139(10):1101–5. doi: 10.1001/archsurg.139.10.1101. [DOI] [PubMed] [Google Scholar]
- 7.Azoulay D, Castaing D, Adam R, Savier E, Delvart V, Karam V, et al. Split-liver transplantation for two adult recipients: feasibility and long-term outcomes. Ann Surg. 2001;233(4):565–74. doi: 10.1097/00000658-200104000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lallier M, St-Vil D, Luks FI, Laberge JM, Bensoussan AL, Guttman FM, et al. Biliary tract complications in pediatric orthotopic liver transplantation. J Pediatr Surg. 1993;28(9):1102–5. doi: 10.1016/0022-3468(93)90139-c. [DOI] [PubMed] [Google Scholar]
- 9.Martelius T, Krogerus L, Hockerstedt K, Bruggeman C, Lautenschlager I. Cytomegalovirus infection is associated with increased inflammation and severe bile duct damage in rat liver allografts. Hepatology. 1998;27(4):996–1002. doi: 10.1002/hep.510270415. [DOI] [PubMed] [Google Scholar]
- 10.Moser MA, Wall WJ. Management of biliary problems after liver transplantation. Liver Transpl. 2001;7(11 Suppl 1):S46–52. doi: 10.1053/jlts.2001.28518. [DOI] [PubMed] [Google Scholar]
- 11.Baccarani U, Isola M, Adani GL, Avellini C, Lorenzin D, Rossetto A, et al. Steatosis of the hepatic graft as a risk factor for post-transplant biliary complications. Clin Transplant. 2010;24(5):631–5. doi: 10.1111/j.1399-0012.2009.01128.x. [DOI] [PubMed] [Google Scholar]
- 12.Evans RW, Manninen DL, Dong FB. An economic analysis of liver transplantation. Costs, insurance coverage, and reimbursement. Gastroenterol Clin North Am. 1993;22(2):451–73. [PubMed] [Google Scholar]
- 13.Engelhardt HT., Jr Shattuck lecture--allocating scarce medical resources and the availability of organ transplantation. Some moral presuppositions. N Engl J Med. 1984;311(1):66–71. doi: 10.1056/NEJM198407053110135. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Urdazpal L, Gores GJ, Ward EM, Maus TP, Buckel EG, Steers JL, et al. Diagnostic features and clinical outcome of ischemic-type biliary complications after liver transplantation. Hepatology. 1993;17(4):605–9. doi: 10.1002/hep.1840170413. [DOI] [PubMed] [Google Scholar]
- 15.Kiuchi T, Ishiko T, Nakamura T, Egawa H, Uemoto S, Inomata Y, et al. Duct-to-duct biliary reconstruction in living donor liver transplantation. Transplant Proc. 2001;33(1–2):1320–1. doi: 10.1016/s0041-1345(00)02490-8. [DOI] [PubMed] [Google Scholar]
- 16.Englesbe MJ, Dimick J, Mathur A, Ads Y, Welling TH, Pelletier SJ, et al. Who pays for biliary complications following liver transplant? A business case for quality improvement. Am J Transplant. 2006;6(12):2978–82. doi: 10.1111/j.1600-6143.2006.01575.x. [DOI] [PubMed] [Google Scholar]
- 17.Kling K, Lau H, Colombani P. Biliary complications of living related pediatric liver transplant patients. Pediatr Transplant. 2004;8(2):178–84. doi: 10.1046/j.1399-3046.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- 18.Gunawansa N, McCall JL, Holden A, Plank L, Munn SR. Biliary complications following orthotopic liver transplantation: a 10-year audit. HPB (Oxford) 2011;13(6):391–9. doi: 10.1111/j.1477-2574.2011.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan ST, Lo CM, Liu CL, Tso WK, Wong J. Biliary reconstruction and complications of right lobe live donor liver transplantation. Ann Surg. 2002;236(5):676–83. doi: 10.1097/00000658-200211000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidenhain C, Pratschke J, Puhl G, Neumann U, Pascher A, Veltzke-Schlieker W, et al. Incidence of and risk factors for ischemic-type biliary lesions following orthotopic liver transplantation. Transpl Int. 2010;23(1):14–22. doi: 10.1111/j.1432-2277.2009.00947.x. [DOI] [PubMed] [Google Scholar]
- 21.Moench C, Moench K, Lohse AW, Thies J, Otto G. Prevention of ischemic-type biliary lesions by arterial back-table pressure perfusion. Liver Transpl. 2003;9(3):285–9. doi: 10.1053/jlts.2003.50015. [DOI] [PubMed] [Google Scholar]
- 22.Theruvath TP, Zhong Z, Pediaditakis P, Ramshesh VK, Currin RT, Tikunov A, et al. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology. 2008;47(1):236–46. doi: 10.1002/hep.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato H, Amersi F, Buelow R, Melinek J, Coito AJ, Ke B, et al. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am J Transplant. 2001;1(2):121–8. [PubMed] [Google Scholar]
- 24.Chavin KD, Taber DJ, Norcross M, Pilch NA, Crego H, McGillicuddy JW, et al. Safe use of highly steatotic livers by utilizing a donor/recipient clinical algorithm. Clin Transplant. 2013;27(5):732–41. doi: 10.1111/ctr.12211. [DOI] [PubMed] [Google Scholar]
- 25.Nativ NI, Maguire TJ, Yarmush G, Brasaemle DL, Henry SD, Guarrera JV, et al. Liver defatting: an alternative approach to enable steatotic liver transplantation. Am J Transplant. 2012;12(12):3176–83. doi: 10.1111/j.1600-6143.2012.04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marty FM, Winston DJ, Rowley SD, Vance E, Papanicolaou GA, Mullane KM, et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med. 2013;369(13):1227–36. doi: 10.1056/NEJMoa1303688. [DOI] [PubMed] [Google Scholar]