Abstract
AIM: To study the effects of total glucosides of peony (TGP) on immunological hepatic fibrosis induced by human albumin in rats.
METHODS: Sixty adult male Sprague-Dawley rats were randomly divided into: Normal group, model group, TGP (60 and 120 mg/kg) treatment groups and colchicines (0.1 mg/kg) treatment group. On the day before the rats were killed, those in TGP or colchicine groups received TGP or colchicine as above from the first day of tail vein injection of human albumin. The rats in normal and model groups were only administered with the same volume of vehicle. At the end of the 16th wk, rats in each group were killed. Blood and tissue specimens were taken. Levels of alanine aminotransferase (ALT), aspartate aminotr-ansferase (AST), nitric oxide (NO), content of malondi-aldehyde (MDA), activity of superoxide dismutase (SOD) and glutathione peroxidase (GSH-px), were measured by biochemical methods. Serum procollagen type III (PC III) and laminin (LN) were determined by radioimmunoassay. Liver collagen level was determined by measuring hydroxyproline content in fresh liver samples. Hepatic tissue sections were stained with hematoxylin-eosin and examined under a light microscope.
RESULTS: Histological results showed that TGP improved the human albumin-induced alterations in the liver structure, alleviated lobular necrosis and significantly lowered collagen content. The antifibrotic effect of TGP was also confirmed by decreased serum content of LN and PCIII in TGP-treated group. Moreover, the treatment with TGP effectively reduced the hydroxyproline content in liver homogenates. However, the level of ALT and AST increased in fibrotic rat but had no significance compared with normal control, whereas the ratio of A/G decreased without significance. TGP had no effect on level of ALT, AST and the ratio of A/G. Furthermore, TGP treatment significantly blocked the increase in MDA and NO, asso-ciated with a partial elevation in liver total antioxidant capacity including SOD and GSH-px.
CONCLUSION: TGP has beneficial effects on hepatic fibrosis in rats by inhibition of collagen synthesis and decreasing oxidative stress.
Keywords: Total glucosides of peony, Hepatic fibrosis, Rat, Oxidative stress
INTRODUCTION
Hepatic fibrosis is traditionally defined as a progressive pathological process involving multiple cellular and molecular events that lead ultimately to deposition of excess matrix proteins in the extracellular space[1-3]. Chronic injury leading to fibrosis in liver occurs in response to a variety of insults, including viral hepatitis (especially hepatitis B in China), alcohol abuse, drugs, metabolic diseases, etc. When this injury process is combined with ineffective regeneration and repair, there is increasing distortion of the normal liver architecture, and the end result is cirrhosis. Current evidence indicates that hepatic fibrosis even cirrhosis is dynamic and can be bidirectional (involving phases of progression and regression)[4,5]. Thus, efforts to understand fibrosis focus primarily on events that lead to the early accumulation of scar in hopes of identifying therapeutic targets to slow its progression. Unfortunately, no effective hepatic antifibrotic therapies are available. Antifibrotic strategies might therefore be usefully targeting towards either reducing matrix synthesis or increasing matrix degradation[6,7].
Paeonia lactiflora pall root, a traditional Chinese herb, has been used to relieve the pain and been a component of effective prescriptions for treatment of liver disease[8]. The total glucosides of peony (TGP), a powder substance extracted from Paeonia lactiflora pall root, were composed of peoniflorin, hydroxypeoniflorin, peonin, albiflorin, benzoylpeoniflorin, etc. Peoniflorin, accounting for some 90%, is a main effective component of TGP. TGP have been recognized as the valuable traditional herbs used in the treatment of rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and hepatitis with a long history in traditional Chinese medicine[9-11]. The anti-inflammatory, anti-oxidative, anti-hepatic injury and immunoregulatory activities without evident toxic or side-effects of TGP have been extensively proved in our laboratory for many years[12-16]. These observations have led to an interest in the potential role of TGP as an antifibrotic agent.
The present study was designed to evaluate whether treatment with TGP exerts any beneficial effect on liver histopathology and liver function in an experimental model of immunological hepatic fibrosis, and the mechanism of its part were also investigated.
MATERIALS AND METHODS
Animal experiments and drug treatment
Sixty adult male Sprague-Dawley rats, weighing 120-150 g [provided by Shanghai BK Experimental Animal Center (Grade II, Certificate No D-65)] were employed in the study. The rats were randomly divided into five groups. A rat model of hepatic fibrosis was produced by immunologically attacking with human albumin, using the method introduced by Wang et al[17]. Ten male Sprague-Dawley rats were regarded as normal group. The other fifty healthy rats were randomly divided into four groups including model group, TGP (60 and 120 mg/kg) treated group and colchicines (0.1 mg/kg) treatment group with the experimental attacking as follows.
All rats were injected with 0.5 mL human albumin diluted with normal saline (0.5 mL equals 4 mg human albumin) and the same quantity of an incomplete Freund’s adjuvant, once every 14 d for the first two times, then once every 10 d, twice. Ten days after the last injection, serum antibody was measured. Rats with positive serum antibody were chosen for experiment through tail vein injection of human albu-min, twice a week, 2.5 mg each in the first week, with a gradual increase of 0.5 mg once each to 4.5 mg eventually, and this dose was maintained for 2 mo. All animals were killed under anesthesia with ether. Blood sample was collected from femoral arteries and veins, centrifuged (3000 r/min for 10 min), and the serum stored at 4 °C until analysis. After this, the liver was quickly washed in situ with ice-cold isotonic saline, removed, and divided into two portions, one was for histological examination, the other immediately frozen in liquid nitrogen.
In this experiment, the animals were divided into five groups randomly which included normal group, model group, TGP (60 and 120 mg/kg) treatment groups and colchicines (0.1 mg/kg) treatment group. The rats in TGP or colchicine groups received TGP or colchicine as above using an 18-gauge stainless steel animal feeding needle from the first day of tail vein injection of human albumin to the day prior to killing the rats. The rats in normal and model groups were fed the same volume of vehicle.
Histopathological examination
Liver tissue sections were fixed in 4 g/L formaldehyde saline and embedded in paraffin. HE staining was performed according to the standard procedure. Histological grade of hepatic fibrosis was determined by a semi-quantitative method based on the criteria of the Knodell index, scoring as the following[18,19], no fibrosis: normal liver and absence of fibrosis; I, perivenular and/or pericellular fibrosis: A few collagen fibrils extended from the central vein and portal tract; II, septal fibrosis: collagen fibrils extension was apparent but had not yet encompassed the whole lobule; III, incomplete cirrhosis: collagen fibrils extended into and encompassed the whole lobule; IV, complete cirrhosis: diffuse extension of collagen fibrils and pseudo-lobule formed. Two pathologists who had no knowledge of their sources examined the stained slides independently. Each sample was observed at 100×magnification and every specimen was analyzed containing a centrilobular vein. The degree of fibrosis was expressed as the mean of 10 different fields in each slide.
Analysis of liver function
The serum activity of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) and the bilirubin concentration were estimated by commercially available kits (Nanjing Jiancheng Institute of Biotechnology, China). Protein conce-ntration was measured according to Lowry et al, using serum bovine albumin as standard and then the ratio of albumin and globulin (A/G) was calculated.
Measurement of NO, LN and PC III in serum
Nitric oxide (NO) content in serum was measured by a microplate assay using Griess reagent, which produces a chromophore with the nitrite[20]. The serum levels of procollagen type III (PC III) and laminin(LN) were determined by radioimmunoassays (Shanghai Navy Medical Institute, China). The operations were performed according to the manufacturer’s instructions.
Measurement of MDA, SOD and GSH-px level in liver homogenates
Livers were thawed, weighed and homogenized with Tris-HCl (5 mmol/L containing 2 mmol/L EDTA, pH 7.4). Homogenates were centrifuged (1000 r/min, 10 min, 4 °C) and the supernatant was used immediately for the assays of MDA,GSH-px and SOD. They were determined following the kit instructions. In brief, MDA in liver tissue was determined by the thiobarbituric acid method[21]. The assays for total SOD and GSH-px were based on their ability to inhibit the oxid-ation of oxyamine by the xanthine-xanthine oxidase system.
Measurement of hydroxyproline content in liver
Liver collagen concentration was determined by measuring hydroxyproline content in fresh liver samples using a modif-ication of the method of Jamall et al[22,23], after digestion with acid, as previously reported. Briefly, liver samples were homogenized and hydrolyzed in 6 N HCl at 110 °C for 18 h. After filtration of the hydrolysate through a 0.45-mm milli-pore filter, chloramine T was added to a final concent-ration of 2.5 mmol/L. The mixture was then treated with 410 mmol/L paradi-methyl-amino-benzaldehyde and incub-ated at 60 °C for 30 min. After cooling to room temper-ature, samples were read spectrophotometrically at 560 nm against a reagent blank containing no tissue and compared with a standard curve of known amount of hydroxyproline. The hydroxyproline content of the liver was expressed as mg/g wet weight.
Statistical analysis
mean±SD were calculated for quantitative data. Significant differences between means were evaluated by analyses of variance and in the case of significance, frequency data were compared using Ridit procedure. A difference was considered significant at P<0.05.
RESULTS
Effect of TGP on liver function of immunological hepatic fibrosis
In model group, the level of ALT and AST increased but had no significant difference compared with normal group, whereas the ratio of A/G decreased also with no significant difference. Transaminase activities in TGP or colchicine treated group tended to decrease, whereas the ratio of A/G had an increasing tendency and both had no significance compared with model group (Table 1).
Table 1.
Effect of TGP on serum ALT, AST activities and A/G value in immunological hepatic fibrosis rat induced by human albumin. (n = 8, mean±SD).
| Groups | Doses (mg/kg) | ALT (U/L) | AST (U/L) | A/G |
| Normal | - | 54.63±22.50 | 61.47±27.81 | 1.17±0.31 |
| Model | - | 78.79±15.03 | 94.51±37.30 | 0.90±0.19 |
| TGP | 60 | 75.67±22.50 | 82.25±17.33 | 0.92±0.21 |
| 120 | 70.61±24.62 | 75.43±26.94 | 0.96±0.17 | |
| Colchicine | 0.1 | 77.71±27.64 | 78.99±22.24 | 0.93±0.18 |
Effect of TGP on hydroxyproline content in liver homogenates
Hepatic fibrosis was quantified by the measurement of hepatic hydroxyproline. It was found that the hydroxyproline content of the model group was significantly higher than that of the normal group. Treatment with TGP or colchicine effectively prevented the immunological hepatic fibrosis induced by human albumin by reducing the hydroxyproline content in liver homogenates (Figure 1).
Figure 1.
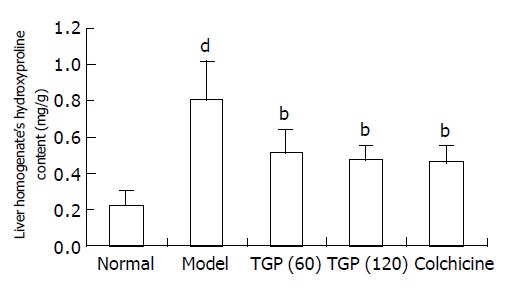
Effects of TGP on contents of hydroxyproline of human albumin-induced fibrotic liver in rats (n = 8, mean±SD) bP<0.01 vs model group; dP<0.01 vs normal control group.
Histological results
From Table 2, we can see the significant difference of patho-logic grading between the normal and model groups. The pathologic grading was significantly decreased in TGP or colchicine treated group.
Table 2.
Effect of TGP on the pathologic grading of immunological hepatic fibrosis rat induced by human albumin. (n = 10, mean±SD).
| Group | Dose(mg/kg) |
Pathologic grading of hepatic fibrosis |
P | ||||
| 0 | I | II | III | IV | |||
| Normal | – | 10 | 0 | 0 | 0 | 0 | – |
| Model | – | 0 | 0 | 3 | 3 | 4 | 0.000d |
| TGP | 60 | 0 | 2 | 5 | 2 | 1 | 0.043a |
| 120 | 0 | 4 | 4 | 2 | 0 | 0.006b | |
| Colchicine | 0.1 | 0 | 3 | 4 | 3 | 0 | 0.016a |
P<0.05,
P<0.01 vs model group;
P<0.01 vs normal control group.
As shown in Figure 2, the structure of liver tissues was normal in control group (Figure 2A). In liver tissues from rats with immunological hepatic fibrosis, hyperplasia of the lattice fibers and collagenous fibers was observed in portal area and extended outwards. Hyperplasia surrounding the central vein observed was distributed along hepatic sinuses and associated with each other. The hepatic lobules were encysted and separated by collagen bundles. The normal structure of lobules was destroyed and pseudolobules formed. Infiltration of small numbers of inflammatory cells was found around the portal area and central vein (Figure 2B). TGP alleviated lobular necrosis and significantly lowered collagen content. The structure of liver tissues was almost normal (Figure 2C). In colchicine-treated group, hyperplasia of the lattice fibers and collagenous fibers was also observed in portal area, but they were alleviated compared with model group (Figure 2D).
Figure 2.
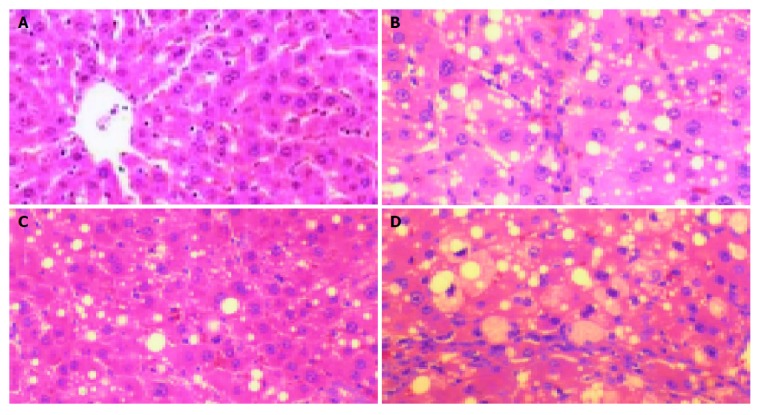
Histological results of tissues stained with HE under light microscope. A: Normal group; B: Model group; C: TGP-treated group; D: Colchicine-treated group.
Effect of TGP on serum LN and PC III
As expected, serum levels of LN and PC III, the surrogate markers of liver fibrogenesis, increased significantly in hepatic fibrotic rats in model group. However, in TGP-treated group they were lower compared with model group. These data confirmed the histological findings that TGP could inhibit hepatic fibrogenesis (Table 3).
Table 3.
Effect of TGP on plasma LN and PC III level in immunological hepatic fibrosis rat induced by human albumin. (n = 8, mean±SD).
| Groups | Doses (mg/kg) | LN (mg/L) | PC III (mg/L) |
| Normal | --- | 106.9±25.4 | 74.4±25.9 |
| Model | --- | 228.0±76.2d | 294.1±99.2d |
| TGP | 60 | 142.1±39.8a | 174.7±69.5 |
| 120 | 127.1±26.8b | 148.5±68.6a | |
| Colchicine | 0.1 | 112.6±27.7b | 121.6±32.6b |
P<0.05,
P<0.01 vs model group;
P<0.01 vs normal control group.
Effect of TGP on MDA content and SOD, GSH-px activities in liver homogenates
Hepatic lipid peroxidation, measured as thiobarbituric acid reactive substance (MDA), was significantly increased in fibrotic rats while liver SOD and GSH-px activities decreased. TGP treatment significantly blocked the increase in MDA and was associated with a partial elevation in liver total antioxidant capacity including SOD and GSH-px (Table 4). In colchicine-treated group, only MDA content was lower than model group.
Table 4.
Effects of TGP on MDA levels, SOD and GSH-px activities liver homogenates in of immunological hepatic fibrotic rats (n = 10, mean±SD).
| Groups | Doses(mg/kg) | MDA (nmol/mg pr) | SOD (U/mg pr) | GSH-Px (U/mg pr) |
| Normal | - | 1.88±0.73 | 142.33±41.00 | 101.00±28.70 |
| Model | - | 4.34±1.12d | 81.95±26.48d | 50.64±15.28d |
| TGP | 60 | 2.41±0.85b | 116.95±32.18 | 73.70±16.60 |
| 120 | 2.21±0.89b | 136.53±36.15a | 83.64±26.16a | |
| Colchicine | 0.1 | 3.02±0.68a | 101.19±44.47 | 69.80±23.65 |
P<0.05,
P<0.01 vs model group;
P<0.01 vs normal control group.
Effect of TGP on NO production in serum
As shown in Figure 3, when the rats were challenged with human albumin, the level of NO was elevated significantly. TGP obviously decreased the NO level while colchicine had no effect.
Figure 3.
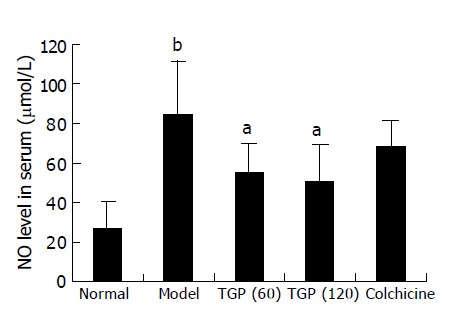
Effects of TGP on NO level in immunological hepatic fibrotic rats (n = 8, mean±SD), aP<0.05 vs model group; bP<0.01 vs normal control group.
DISCUSSION
Hepatic fibrosis is the common consequence of chronic liver injury of any etiology. Advanced hepatic fibrosis disrupts the normal liver architecture, causing hepatocellular dysfunction and portal hypertension. It is of great significance to search for effective ways to inhibit fibrogenesis and prevent the development of cirrhosis[24]. Unfortunately, no effective hepatic antifibrotic therapies are available. Colchicine has been commonly used for anti-fibrosis, but its side effect is severe and its clinical application is limited. Medicinally useful plants including traditional Chinese herbs are well known for their cheap prices and negligible side effects and have particular potential in the treatment of hepatic fibrosis[25,26]. In this study we firstly established the animal model of immunological hepatic fibrosis. The histological results showed that the normal structure of lobules was destroyed and pseudolobules formed, which were similar to the pathology of chemical hepatic fibrosis induced by long-term admini-stration of carbon tetrachloride. Moreover, LN and PC III are known to be good serum markers of hepatic fibrog-enesis, thus the increased hydroxyproline content in liver and serum LN and PC III also confirmed the hepatic fibrogenesis in rats. Unlike the severe hepatocyte necrosis and inflam-mation induced by CCl4 toxicity, the ALT or AST released from hepatocytes did not increase and less immigration of inflammatory cells in liver indicated the mild inflammation in rat with immunological hepatic fibrosis induced by human albumin. Those results are in accordance with the findings of immunological hepatic fibrogenesis[17]. The present study demonstrated that administration of TGP was effective in treating hepatic fibrosis in rats based on both histological examination and functional analysis. The results obtained provide a basis for further studies on the potentially protective effect of TGP on liver function in cirrhotic patients.
Increasing experimental evidence suggests that reactive oxygen species (ROS) such as H2O2, O·2-, and OH•, are implicated in the development and pathological progress of hepatic fibrosis[27-30]. Under normal conditions, low amounts of ROS are produced as by-products of the aerobic respiration. At high doses, ROS are noxious to the cells leading to impaired metabolic functions, growth inhibition, and ultimately cell death. Cells therefore employ several antioxidant enzyme systems to maintain low levels of ROS, which plays a key role in hepatic fibrosis[29]. Increased ROS and resulting oxidative stress are commonly detected in livers from patients with alcohol abuse, hepatitis C virus infection, iron overload, or chronic cholestasis, as well as in most types of experimental liver fibrogenesis[31]. Oxidative stress, in particular, lipid peroxidation, induces collagen synthesis. It also acts as a signaling mediator for transforming growth factor (TGF)-β, and plays a major role in hepatic fibrosis. Hepatic stellate cells produce and respond to TGF-β in an autocrine manner with increased collagen expression. Consequently, antioxidants, particularly those of plant origin, have emerged as potent antifibrotic agents. Previous and recent findings on the antifibrotic potential of plant-derived antioxidants could attenuate hepatic fibrosis in rodents and may exert beneficial effects in patients with chronic liver diseases.
Our previous study showed that TGP attenuated inflam-mation and ROS and TGP inhibited hydrogen peroxide (H2O2) were released from peritoneal macrophages in adjuvant arthritis (AA) rats[15]. It was also found that treatment of AA rats with TGP (50 mg/kg) ig (14-28 d) could counteract the elevated level of MDA and NO and the lowered activities of SOD and GSH-px. The hemolytic action of H2O2 is related to the induction of lipid perox-idation and glutathione depletion in human erythrocytes. TGP (0.5-2.5 mg/L) could significantly inhibit hemolysis, lipid peroxidation, and glutathione depletion induced by H2O2. In vitro, TGP could scavenge OH·and O·2-[9-15]. In the experimental model of human albumin-induced fibrosis, hepatic injury occurs with an increased generation of ROS that causes lipid peroxidation. In addition to being a product of lipid peroxidation, oxidative stress may result from deran-gement of antioxidant defenses including SOD and decr-eased GSH-px activities. Inverse correlations between antioxidant enzymes and pathology scores and/or lipid pero-xidation have been found in rats with CCl4-induced cirrhosis, biliary obstruction, or alcoholic liver disease[30-32]. Beneficial effects and diminished hepatic fibrosis by TGP may be partially related to the preservation of antioxidant enzymes defenses and reduction of lipid peroxidation, but colchicine treatment only significantly inhibited malondialdehyde[33]. Changes in nitric oxide (NO) production may also play a role in the TGP-induced prevention of liver damage in cirrhotic rats. NO has been shown to react with superoxide generating the strong oxidant peroxynitrite, which can initiate lipid peroxidation or cause a direct inhibition of the mitoch-ondrial respiratory chain[34]. TGP was found to inhibit prod-uction of NO in this human albumin induced immun-ological hepatic fibrotic rats.
In conclusion, TGP could greatly retard the progression of experimental immunological hepatic fibrosis through inhibition of collagen synthesis and decreasing oxidative stress. Therefore, it is a potentially new antifibrotic drug for clinical application.
Footnotes
Supported by the National High Technology Research and Development Program of China (863 Program), No. 2002AA2Z3235
References
- 1.Dai WJ, Jiang HC. Advances in gene therapy of liver cirrhosis: a review. World J Gastroenterol. 2001;7:1–8. doi: 10.3748/wjg.v7.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 4.Mann DA, Smart DE. Transcriptional regulation of hepatic stellate cell activation. Gut. 2002;50:891–896. doi: 10.1136/gut.50.6.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonis PA, Friedman SL, Kaplan MM. Is liver fibrosis reversible? N Engl J Med. 2001;344:452–454. doi: 10.1056/NEJM200102083440610. [DOI] [PubMed] [Google Scholar]
- 6.Albanis E, Friedman SL. Hepatic fibrosis. Pathogenesis and principles of therapy. Clin Liver Dis. 2001;5:315–334, v-vi. doi: 10.1016/s1089-3261(05)70168-9. [DOI] [PubMed] [Google Scholar]
- 7.Yang XB, Huang ZM, Wang JH. The drug therapy of liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2002;10:956–957. [Google Scholar]
- 8.Dai LM, Chen MZ, Xu SY. Protective effects of total glucocides of paeony on experimental hepatitis. Zhongguo Yaolixue Tongbao. 1993;9:449–453. [Google Scholar]
- 9.Wang B, Yao YY, Zhou AW, Ge ZD, Chen MZ, Xu SY. Protective effect of total glucosides of paeony on joint damage in adjuvant arthritis rats. Zhongguo Yaolixue Yu Dulixue Zazhi. 1996;10:211–214. [Google Scholar]
- 10.Wang B, Yao YY, Zhou AW, Chen MZ, Xu SY. Study on immunomodulatory effect of total glucosides of paeony and its relationship with nitric oxide in adjuvant arthritis rats. Zhongguo Mianyixue Zazhi. 1996;12:104–106. [Google Scholar]
- 11.Zhou LL, Wei W, Shen YX, Xu SY. The effects of TGP on CJ-S131-induced systemic lupus erythematosus-like model in mice. Zhongguo Yaolixue Tongbao. 2002;18:175–177. [Google Scholar]
- 12.Xu SY, Shen YX, Xu DJ, Wei W, Liang JS. Effects of total glucosides of paeony and moutan cortex on the function of pineal gland in inflammatory-immune regulation in rats. Zhongguo Yaolixue Yu Dulixue Zazhi. 1994;8:161–165. [Google Scholar]
- 13.Wei W, Liang JS, Zhou AW, Chen MZ, Xu SY. Effects of total glucosides of paeony on interleukin 2 production. Zhongguo Yaolixue Tongbao. 1989;5:176–179. [Google Scholar]
- 14.Ge ZD, Wei W, Shen YX, Wang B, Ding CH, Zhou AW, Zhang AP, Xu SY. Effects of paeoniflorin, total glucosides of paeony removed paeoniflorin on interleukin 2 production by splenic lymphocytes form adjuvant arthritic rats. Anhui Yike Daxue Xuebao. 1996;31:4–6. [Google Scholar]
- 15.Gao BB, Dai LM, Xu SY. The scavenging activities of TGM and TGP on free radicals. Suifang Yixueyuan Xuebao. 1996;18:43–46. [Google Scholar]
- 16.Wang B, Chen MZ, Xu SY. Regulatory mechanism of total glucosides of paeony on tumor necrosis factor production by peritoneal macrophages in rats. Zhongguo Yaolixue Tongbao. 1997;13:255–257. [Google Scholar]
- 17.Wang BE, Wang ZF, Yin WY, Huang SF, Li JJ. Study on expe-rimental immunity model of hepatic fibrosis. Zhonghua Yixue Zazhi. 1998;69:503–505. [Google Scholar]
- 18.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 19.Chevallier M, Guerret S, Chossegros P, Gerard F, Grimaud JA. A histological semiquantitative scoring system for evaluation of hepatic fibrosis in needle liver biopsy specimens: comparison with morphometric studies. Hepatology. 1994;20:349–355. [PubMed] [Google Scholar]
- 20.Kiechle FL, Malinski T. Nitric oxide. Biochemistry, pathophysiology, and detection. Am J Clin Pathol. 1993;100:567–575. doi: 10.1093/ajcp/100.5.567. [DOI] [PubMed] [Google Scholar]
- 21.Gavino VC, Miller JS, Ikharebha SO, Milo GE, Cornwell DG. Effect of polyunsaturated fatty acids and antioxidants on lipid peroxidation in tissue cultures. J Lipid Res. 1981;22:763–769. [PubMed] [Google Scholar]
- 22.Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;112:70–75. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 23.Fort J, Pilette C, Veal N, Oberti F, Gallois Y, Douay O, Rosenbaum J, Calès P. Effects of long-term administration of interferon alpha in two models of liver fibrosis in rats. J Hepatol. 1998;29:263–270. doi: 10.1016/s0168-8278(98)80012-3. [DOI] [PubMed] [Google Scholar]
- 24.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu I, Ma YR, Mizobuchi Y, Liu F, Miura T, Nakai Y, Yasuda M, Shiba M, Horie T, Amagaya S, et al. Effects of Sho-saiko-to, a Japanese herbal medicine, on hepatic fibrosis in rats. Hepatology. 1999;29:149–160. doi: 10.1002/hep.510290108. [DOI] [PubMed] [Google Scholar]
- 26.Li BS, Wang J, Zhen YJ, Liu JX, Wei MX, Sun SQ, Wang SQ. Experimental study on serum fibrosis markers and liver tissue pathology and hepatic fibrosis in immuno-damaged rats. Shijie Huaren Xiaohua Zazhi. 1999;7:1031–1034. [Google Scholar]
- 27.Apte M. Oxidative stress: does it 'initiate' hepatic stellate cell activation or only 'perpetuate' the process? J Gastroenterol Hepatol. 2002;17:1045–1048. doi: 10.1046/j.1440-1746.2002.02845.x. [DOI] [PubMed] [Google Scholar]
- 28.Serviddio G, Pereda J, Pallardó FV, Carretero J, Borras C, Cutrin J, Vendemiale G, Poli G, Viña J, Sastre J. Ursodeoxycholic acid protects against secondary biliary cirrhosis in rats by preventing mitochondrial oxidative stress. Hepatology. 2004;39:711–720. doi: 10.1002/hep.20101. [DOI] [PubMed] [Google Scholar]
- 29.Muriel P, Moreno MG. Effects of silymarin and vitamins E and C on liver damage induced by prolonged biliary obstruction in the rat. Basic Clin Pharmacol Toxicol. 2004;94:99–104. doi: 10.1111/j.1742-7843.2004.pto940207.x. [DOI] [PubMed] [Google Scholar]
- 30.Lu G, Shimizu I, Cui X, Itonaga M, Tamaki K, Fukuno H, Inoue H, Honda H, Ito S. Antioxidant and antiapoptotic activities of idoxifene and estradiol in hepatic fibrosis in rats. Life Sci. 2004;74:897–907. doi: 10.1016/j.lfs.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Pietrangelo A. Iron-induced oxidant stress in alcoholic liver fibrogenesis. Alcohol. 2003;30:121–129. doi: 10.1016/s0741-8329(03)00126-5. [DOI] [PubMed] [Google Scholar]
- 32.Gebhardt R. Oxidative stress, plant-derived antioxidants and liver fibrosis. Planta Med. 2002;68:289–296. doi: 10.1055/s-2002-26761. [DOI] [PubMed] [Google Scholar]
- 33.Das D, Pemberton PW, Burrows PC, Gordon C, Smith A, McMahon RF, Warnes TW. Antioxidant properties of colchicine in acute carbon tetrachloride induced rat liver injury and its role in the resolution of established cirrhosis. Biochim Biophys Acta. 2000;1502:351–362. doi: 10.1016/s0925-4439(00)00059-4. [DOI] [PubMed] [Google Scholar]
- 34.Koruk M, Aksoy H, Akçay F, Onuk MD. Antioxidant capacity and nitric oxide in patients with hepatic cirrhosis. Ann Clin Lab Sci. 2002;32:252–256. [PubMed] [Google Scholar]


