Abstract
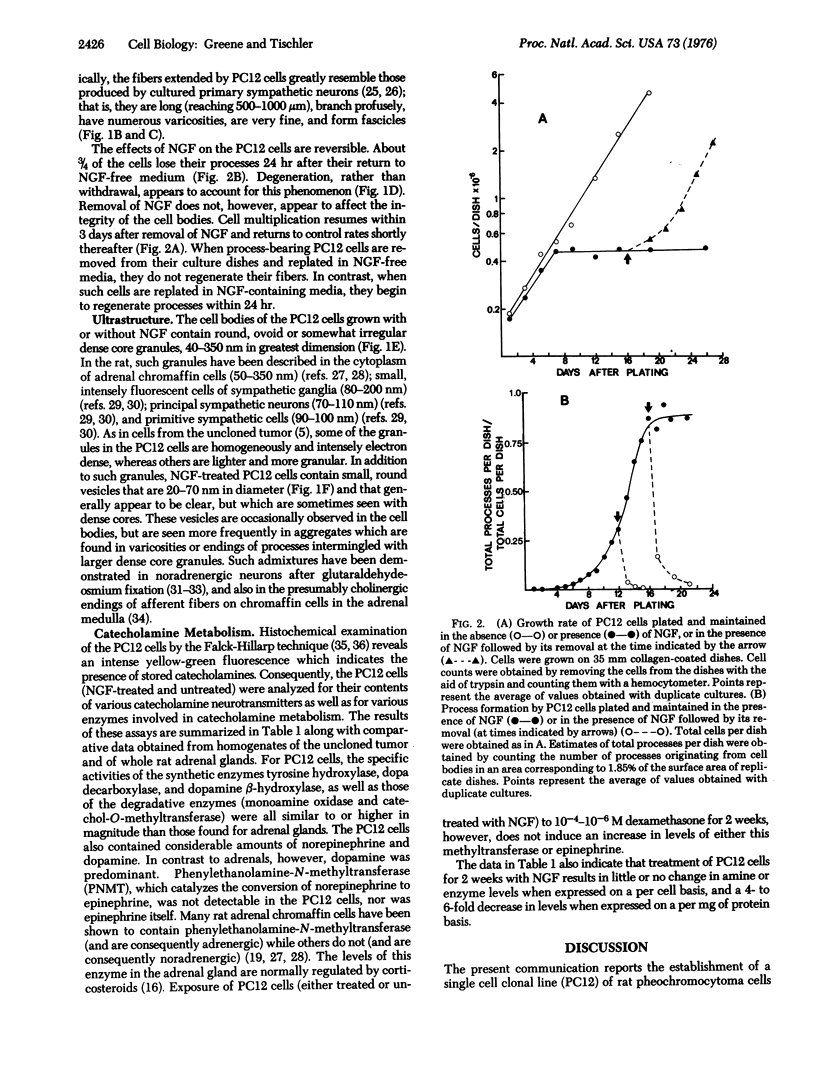
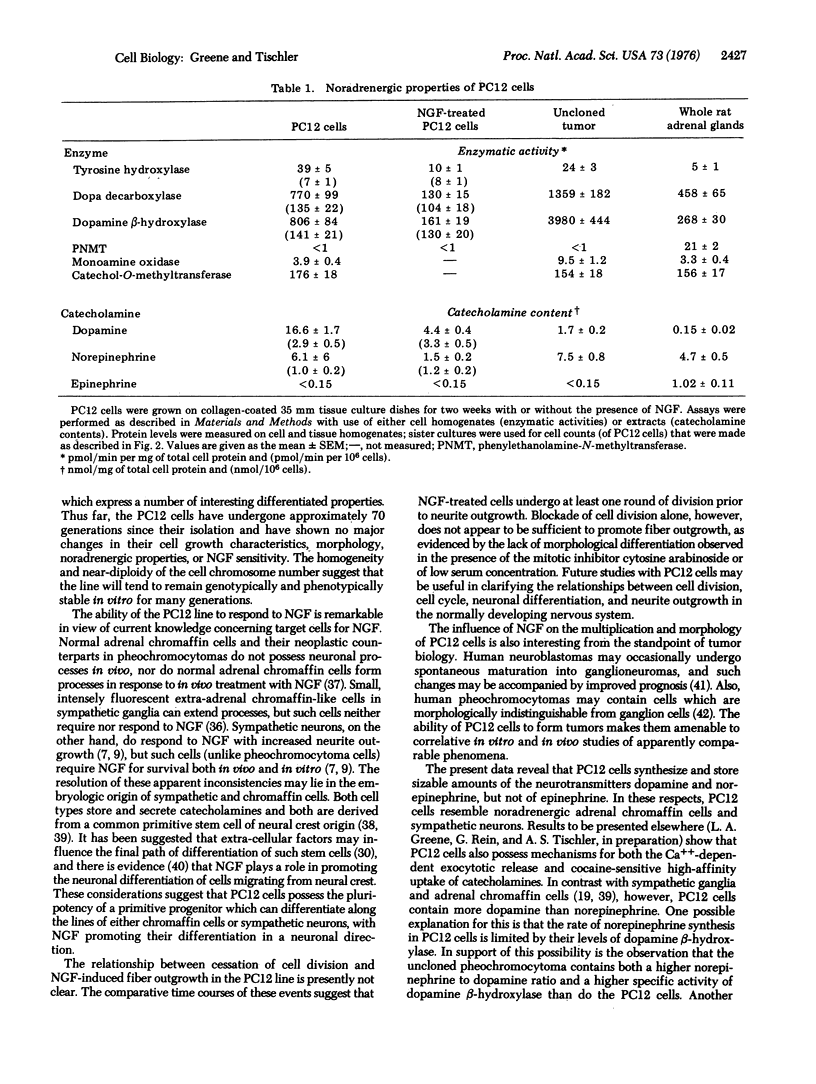
A single cell clonal line which responds reversibly to nerve growth factor (NGF) has been established from a transplantable rat adrenal pheochromocytoma. This line, designated PC12, has a homogeneous and near-diploid chromosome number of 40. By 1 week's exposure to NGF, PC12 cells cease to multiply and begin to extend branching varicose processes similar to those produced by sympathetic neurons in primary cell culture. By several weeks of exposure to NGF, the PC12 processes reach 500-1000 mum in length. Removal of NGF is followed by degeneration of processes within 24 hr and by resumption of cell multiplication within 72 hr. PC12 cells grown with or without NGF contain dense core chromaffin-like granules up to 350 nm in diameter. The NGF-treated cells also contain small vesicles which accumulate in process varicosities and endings. PC12 cells synthesize and store the catecholamine neurotransmitters dopamine and norepinephrine. The levels (per mg of protein) of catecholamines and of the their synthetic enzymes in PC12 cells are comparable to or higher than those found in rat adrenals. NGF-treatment of PC12 cells results in no change in the levels of catecholamines or of their synthetic enzymes when expressed on a per cell basis, but does result in a 4- to 6-fold decrease in levels when expressed on a per mg of protein basis. PC12 cells do not synthesize epinephrine and cannot be induced to do so by treatment with dexamethasone. The PC12 cell line should be a useful model system for neurobiological and neurochemical studies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angeletti P. U., Levi-Montalcini R., Kettler R., Thoenen H. Comparative studies on the effect of the nerve growth factor on sympathetic ganglia and adrenal medulla in newborn rats. Brain Res. 1972 Sep 15;44(1):197–206. doi: 10.1016/0006-8993(72)90375-7. [DOI] [PubMed] [Google Scholar]
- BORNSTEIN M. B. Reconstituted rattail collagen used as substrate for tissue cultures on coverslips in Maximow slides and roller tubes. Lab Invest. 1958 Mar-Apr;7(2):134–137. [PubMed] [Google Scholar]
- Bjerre B., Björklund A. The production of catecholamine-containing cells in vitro by young chick embryos: effects of "nerve growth factor" (NGF) and its antiserum. Neurobiology. 1973;3(3):140–161. [PubMed] [Google Scholar]
- Black I. B., Hendry I., Iversen L. L. Differences in the regulation of tyrosine hydroxylase and dopa decarboxylase in sympathetic ganglia and adrenals. Nat New Biol. 1971 May 5;231(18):27–29. [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUPLAND R. E. (ELECTRON MICROSCOPIC OBSERVATIONS ON THE STRUCTURE OF THE RAT ADRENAL MEDULLA. I. THE ULTRASTRUCTURE AND ORGANIZATION OF CHROMAFFIN CELLS IN THE NORMAL ADRENAL MEDULLA.) J Anat. 1965 Apr;99:231–254. [PMC free article] [PubMed] [Google Scholar]
- Chamley J. H., Mark G. E., Campbell G. R., Burnstock G. Sympathetic ganglia in culture. I. Neurons. Z Zellforsch Mikrosk Anat. 1972;135(3):287–314. doi: 10.1007/BF00307178. [DOI] [PubMed] [Google Scholar]
- Contributions of clonal systems to neurobiology. Neurosci Res Program Bull. 1973 Dec;11(5):412–536. [PubMed] [Google Scholar]
- Coupland R. E. Electron microscopic observations on the structure of the rat adrenal medulla: II. Normal innervation. J Anat. 1965 Apr;99(Pt 2):255–272. [PMC free article] [PubMed] [Google Scholar]
- DeLellis R. A., Merk F. B., Deckers P., Warren S., Balogh K. Ultrastructure and in vitro growth characteristics of a transplantable rat pheochromocytoma. Cancer. 1973 Jul;32(1):227–235. doi: 10.1002/1097-0142(197307)32:1<227::aid-cncr2820320134>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- FOX F., DAVIDSON J., THOMAS L. B. Maturation of sympathicoblastoma into ganglioneuroma; report of 2 patients with 20-and 46-year survivals respectively. Cancer. 1959 Jan-Feb;12(1):108–116. doi: 10.1002/1097-0142(195901/02)12:1<108::aid-cncr2820120116>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Geffen L. B., Livett B. G. Synaptic vesicles in sympathetic neurons. Physiol Rev. 1971 Jan;51(1):98–157. doi: 10.1152/physrev.1971.51.1.98. [DOI] [PubMed] [Google Scholar]
- Greene L. A. A dissociated cell culture bioassay for nerve growth factor. Neurobiology. 1974;4(5):286–292. [PubMed] [Google Scholar]
- Grillo M. A. Electron microscopy of sympathetic tissues. Pharmacol Rev. 1966 Mar;18(1):387–399. [PubMed] [Google Scholar]
- Holtzman E., Teichberg S., Abrahams S. J., Citkowitz E., Crain S. M., Kawai N., Peterson E. R. Notes on synaptic vesicles and related structures, endoplasmic reticulum, lysosomes and peroxisomes in nervous tissue and the adrenal medulla. J Histochem Cytochem. 1973 Apr;21(4):349–385. doi: 10.1177/21.4.349. [DOI] [PubMed] [Google Scholar]
- Jacobowitz D. M., Greene L. A. Histofluorescence study of chromaffin cells in dissociated cell cultures of chick embryo sympathetic ganglia. J Neurobiol. 1974;5(1):65–83. doi: 10.1002/neu.480050107. [DOI] [PubMed] [Google Scholar]
- Kanerva L. Ultrastructure of sympathetic ganglion cells and granule-containing cells in the paracervical (Frankenhäuser) ganglion of the newborn rat. Z Zellforsch Mikrosk Anat. 1972;126(1):25–40. doi: 10.1007/BF00306778. [DOI] [PubMed] [Google Scholar]
- Kato T., Kuzuya H., Nagatsu T. A simple and sensitive assay for dopamine-beta-hydroxylase activity by dual-wavelength spectrophotometry. Biochem Med. 1974 Aug;10(4):320–328. doi: 10.1016/0006-2944(74)90035-0. [DOI] [PubMed] [Google Scholar]
- Kolber A. R., Goldstein M. N., Moore B. W. Effect of nerve growth factor on the expression of colchicine-binding activity and 14-3-2 protein in an established line of human neuroblastoma. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4203–4207. doi: 10.1073/pnas.71.10.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R., ANGELETTI P. U. Essential role of the nerve growth factor in the survival and maintenance of dissociated sensory and sympathetic embryonic nerve cells in vitro. Dev Biol. 1963 Mar;6:653–659. doi: 10.1016/0012-1606(63)90149-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Patterson P. H. Primary cultures of dissociated sympathetic neurons. I. Establishment of long-term growth in culture and studies of differentiated properties. J Cell Biol. 1973 Nov;59(2 Pt 1):329–345. doi: 10.1083/jcb.59.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains R. E., Patterson P. H. Primary cultures of dissociated sympathetic neurons. II. Initial studies on catecholamine metabolism. J Cell Biol. 1973 Nov;59(2 Pt 1):346–360. doi: 10.1083/jcb.59.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaman R. E. Microdetermination of catechol-O-methyl transferase in brain. Life Sci. 1965 Dec;4(24):2353–2359. doi: 10.1016/0024-3205(65)90290-0. [DOI] [PubMed] [Google Scholar]
- Morton H. J. A survey of commercially available tissue culture media. In Vitro. 1970 Sep-Oct;6(2):89–108. doi: 10.1007/BF02616112. [DOI] [PubMed] [Google Scholar]
- Schubert D., Heinemann S., Carlisle W., Tarikas H., Kimes B., Patrick J., Steinbach J. H., Culp W., Brandt B. L. Clonal cell lines from the rat central nervous system. Nature. 1974 May 17;249(454):224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Sumner A. T., Evans H. J., Buckland R. A. New technique for distinguishing between human chromosomes. Nat New Biol. 1971 Jul 7;232(27):31–32. doi: 10.1038/newbio232031a0. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Angeletti P. U., Levi-Montalcini R., Kettler R. Selective induction by nerve growth factor of tyrosine hydroxylase and dopamine- -hydroxylase in the rat superior cervical ganglia. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1598–1602. doi: 10.1073/pnas.68.7.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler A. S., Greene L. A. Nerve growth factor-induced process formation by cultured rat pheochromocytoma cells. Nature. 1975 Nov 27;258(5533):341–342. doi: 10.1038/258341a0. [DOI] [PubMed] [Google Scholar]
- Uretsky N. J., Iversen L. L. Effects of 6-hydroxydopamine on catecholamine containing neurones in the rat brain. J Neurochem. 1970 Feb;17(2):269–278. doi: 10.1111/j.1471-4159.1970.tb02210.x. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Sytkowski A. J., Nirenberg M. W. Acetylcholine receptors of muscle grown in vitro. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3180–3184. doi: 10.1073/pnas.69.11.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WURTMAN R. J., AXELROD J. A SENSITIVE AND SPECIFIC ASSAY FOR THE ESTIMATION OF MONOAMINE OXIDASE. Biochem Pharmacol. 1963 Dec;12:1439–1441. doi: 10.1016/0006-2952(63)90215-6. [DOI] [PubMed] [Google Scholar]
- Warren S., Chute R. N. Pheochromocytoma. Cancer. 1972 Feb;29(2):327–331. doi: 10.1002/1097-0142(197202)29:2<327::aid-cncr2820290210>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Weston J. A. The migration and differentiation of neural crest cells. Adv Morphog. 1970;8:41–114. doi: 10.1016/b978-0-12-028608-9.50006-5. [DOI] [PubMed] [Google Scholar]
- Wurtman R. J., Axelrod J. Control of enzymatic synthesis of adrenaline in the adrenal medulla by adrenal cortical steroids. J Biol Chem. 1966 May 25;241(10):2301–2305. [PubMed] [Google Scholar]




