Abstract
AIM: Ischemia/reperfusion (I/R) injury is one of the major obstacles for intestinal transplantation (ITx). Urinary trypsin inhibitor (Ulinastatin, UTI) suppresses proteases and stabilizes lysosomal membranes. We supposed that Ulinastatin would diminish I/R injury of intestinal graft.
METHODS: UTI- treated group and untreated control group were investigated by histological assessment at 1.5, 4, 24, and 72 h after ITx. Myeloperoxidase (MPO) activity was used as the activity of neutrophils, and malondialdehyde (MDA) was used as an index of lipid peroxidation. TNFα and i-NOS mRNA expression in graft tissue were measured by semi-quantitative RT-PCR. CD11b+ Gr1+ cells in graft lamina propria were analyzed by flow cytometry.
RESULTS: Histological scores of the graft showed that the tissue injury was markedly attenuated by UTI treatment at different time points after ITx, with reduced MPO and MDA value in the grafts. The expression of TNFα and i-NOS mRNA was profoundly inhibited, while the infiltration of CD11b+ Gr1+ cells into the intestinal graft was decreased in UTI group.
CONCLUSION: Urinary trypsin inhibitor attenuates I/R injury in mouse intestinal transplantation by reducing monocytes infiltration and down-regulation of TNFα and i-NOS mRNA expression.
Keywords: Ischemia/reperfusion injury, Ulinastatin, Intestinal transplantation
INTRODUCTION
Intestinal transplantation (ITx) is the only definitive therapy for patients with irreversible intestinal failure who have developed life-threatening complications of total parenteral nutrition[1]. The outcome of clinical ITx, when compared to other solid organ transplants, is still far from acceptable[2]. Ischemia/reperfusion (I/R) injury has been one of the major obstacles, since the intestinal graft is exceedingly sensitive to ischemia caused by storage and implantation[3]. I/R injury subsequently leads to impairment of mucosal barrier and bacterial translocation, which provoke graft loss, fatal infection, and even multi-organ dysfunction in recipients. Therefore, more effective protocols for controlling graft I/R injury are demanded, in order to achieve a better outcome for this therapeutic approach.
Urinary trypsin inhibitor (Ulinastatin, UTI), a glycoprotein with a molecular weight of 67000, is a protease inhibitor purified from human urine. It suppresses proteases such as trypsin, chymotrypsin and elastase, as well as stabilizes lysosomal membranes and thereby inhibits the release of lysosomal enzymes[4]. Ulinastatin has been found effective in relieving reperfusion injury in ischemic liver[5], intestine[6], and kidney[7]. In our knowledge, however, its effect on intestinal transplantation has not been reported. Distinct from other causes, the ischemic event in transplantation is inevitable, featured by a shorter warm ischemic and a longer cold ischemic period[3]. In our previous studies, we had found quick influx of monocytes, mainly neutrophils and macrophages, into intestinal graft even in syngeneic combination. I/R injury is relevant to the accumulation and eruption of these infiltrating monocytes. We supposed that the suppressive effects on neutrophil protease by Ulinastatin would diminish I/R injury of intestinal graft. In the present study, we examined the effects of Ulinastatin on I/R injury of intestinal grafts and the expression of TNFα and i-NOS mRNA in a mouse intestinal transplantation model.
MATERIALS AND METHODS
Animals
Balb/c (H-2d) mice were purchased from The Central Animal Facility of Medical School, Zhejiang University, and were used at 8-12 wk of age. All animals were housed under specific pathogen-free conditions with controlled light/dark cycles and free access to water and rodent chow. The investigations were performed in compliance with the policies of the animal care committee of the local government.
Heterotopic intestinal transplantation in mice
Mouse heterotopic intestinal transplantation was performed as described by Zhong et al[8] with minor modifications. Balb/c (H-2d) mice were used as donors and recipients. Briefly, ITx was carried out under intraperitoneal anesthesia using ketamine (0.08 mg/g) and xylazine (0.012 mg/g). First, the donor jejunum and proximal part of the ileum were isolated with attached superior mesenteric artery and portal vein. After luminal irrigation and vascular perfusion, the graft was harvested and stored in 4 °C Ringer’s solution until implantation. Graft portal vein and superior mesenteric artery were anastomosed to recipient’s inferior vena cava and aorta in an end-to-side fashion. The distal end of the intestinal graft was connected to the host jejunum. The proximal graft lumen was exteriorized as a stoma. No antibiotics were administered perioperatively.
Experimental groups and peri-transplant therapy
Three experimental groups were established: Group 1, Sham operation (n = 6): Laparotomy and dissection of SMA and PV were performed without occlusion of these vessels in this group. Group 2, UTI-treated group (n = 24): UTI (50000 U/kg, Techpool Bio-Pharma Co. Guangzhou, China) was given to the recipients via intravenous injection 5 min before revascularization of the intestinal graft. Thereafter, it was given at the same level of dose every 12 h after transplantation. Group 3, untreated controls (n = 24): Normal saline instead of UTI was given to the recipients. At 1.5, 4, 24, 72 h after transplantation, six mice of each group were killed and the grafts were harvested for histological grading, FACS analysis, and mRNA extraction.
Histological assessment
A 3-cm segment of the proximal portion of the graft was divided along its anti-mesenteric side and washed in PBS. The samples were fixed by formalin and embedded in paraffin by Swiss rolls with the luminal side facing outwards. Then, the rolled samples were cross-sectioned and stained with hemotoxylin-eosin. All microscopic slides were observed and assessed blindly with reference to the histological criteria. Briefly, specimens were scored as: (0) less than 50% of villous tips damaged, (1) more than 50% of villous tips damaged; (2) damage confined to distal 1/3 of villus; (3) damage confined to distal 2/3 of villus; (4) damage including all of villus, and (5) damage more proximal than villus.
Assay of myeloperoxidase (MPO) activity
MPO activity was assayed by spectrophotometrically measuring the H2O2 dependent oxidation of 3,3’,5,5’-tetramethylbenzidine at 650 nm. All the reagents in the assay were afforded by a MPO kit, obtained from Jiancheng Bio-technology, Nanjing, PR China.
Assay of malondialdehyde (MDA)
The lipid peroxide products, malondialdehyde (MDA) was used as an index of lipid peroxidation and was expressed as nmol/g protein. It was determined by TBA assay, using a MDA kit obtained from Jiancheng Bio-technology, Nanjing, PR China.
Measurement of TNFα and i-NOS mRNA by semiquantitative RT-PCR
Graft RNA was extracted by Trizol kit from each 50-100 mg snap-frozen samples. The concentration and A260/A280 (>1.8) was determined by ultraviolet photospectrometry. Total RNA (4 μg) was reverse-transcribed to c-DNA and expanded by PCR. The primers of TNFα, i-NOS and β-actin are as follows:
TNFα:Primer 1: 5’-AGCCCACGTAGCAAACCACCAA-3’
Primer 2: 5’-ACACCCATTCCCTTCACAGAGC AAT-3’
i-NOS: Primer 1: 5’-ACCCCTGTCTTCCACCAGGAGTTGAA-3’
Primer 2: 5’-TGCAGCCATGACCTTTCGCATTAGCATGG-3’
β-actin: Primer 1: 5’-TGGAATCCTGTGGCATCCATGAAAC-3’
Primer 2: 5’-TAAAACGCAGCTCAGTAACAGTCCG-3’
The expanded products were analyzed by 1.5% agarose gel electrophoresis and imaged by Kodak digital imaging system. The relative value of mRNA was determined by the ratio between the A of target gene and β-actin.
Flow cytometry
Three-color flow cytometry was conducted on isolated cells from the lamina propria of the intestinal grafts. Isolated cells from each preparation were centrifuged at 2000 r/min for 3 min, then washed twice in FACS buffer (PBS+1% FCS+5% normal rat serum) and incubated with primary mAbs for 20 min at 4 °C. The specimens incubated with biotinylated mAbs were subsequently developed with streptavidin PercP. After washing twice, all samples were analyzed on a FACScan® cytometer (Becton Dickinson). 10000 to 20000 events were collected from each sample. Data analysis was performed using WinMDI 2.8 software.
Statistical analysis
Histology score, and MPO, MDA, i-NOS/β-actin,TNFα/β-actin value, were calculated for each group. Student’s t- test was used to determine the significance of differences in means. The non-parametric Mann-Whitney U-test was used to compare the medians of groups that did not follow a normal distribution according to the Shapiro-Witt test. A P-value of less than 0.05 was considered significant. SPSS 12.0 and Microsoft Excel software were used for the statistical analyses.
RESULTS
Histological finding of the intestinal grafts
The histological scores of the intestinal grafts in both control and UTI groups were shown in Figure 1 (A-D). At different time points after transplantation, the histological impairments in UTI group were apparently attenuated compared to the control group. At the early stage after reperfusion (1.5, 4, and 24 h after transplantation), the mucosal damage in the UTI group is confined to villous tips, obviously slighter than the control group, in which the damage developed in total villi. The histological changes nearly disappeared after 72 h in the UTI group, whereas villous impairments remained in the distal part of the villus in the control group. Moderate congestion and marked edema of the intestinal graft at early time point were found in control groups. In the UTI group, however, no congestion was found and the tissue edema was slighter. Comparing with the control group, the infiltration of monocytes was also reduced in UTI group at each time point after transplantation by light microscopic observation.
Figure 1.
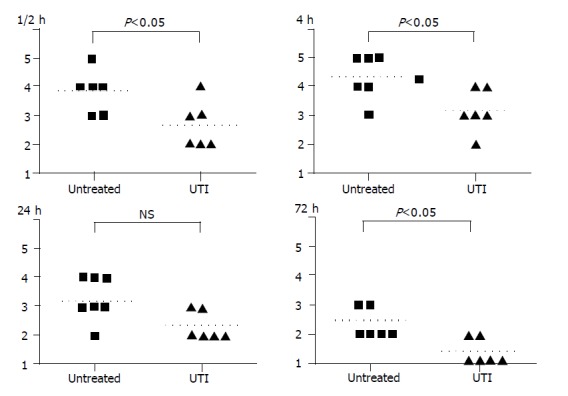
At 1.5, 4, 24, and 72 h after intestinal transplantation, the histological scores of tissue injury in the UTI-treated group were apparently less than the untreated group.
Myeloperoxidase (MPO) activity
As shown in Figure 2, the MPO activity of the intestinal graft tissue in the UTI group remained at a lower level when compared to the control group in the earlier time points after I/R (0.46±0.23 vs 0.63±0.17, 1.05±0.12 vs 1.25±0.15, 0.65±0.13 vs 0.85±0.18 at 1.5, 4, and 24 h after transplantation respectively, U/g, n = 6 for each group, P<0.05). At 24 h after transplantation, the MPO activity in both UTI and control groups were reverted to 0.69±0.14 U/g and 0.85±0.15 U/g, no statistical difference were found in this time point.
Figure 2.
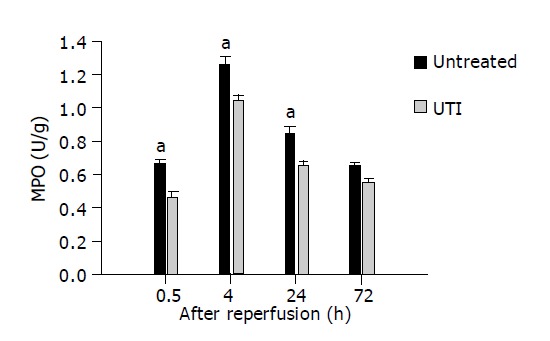
MPO activity in the intestinal grafts in the UTI-treated group remained at a lower level when compared with the control group in the earlier time points after IR (1.5, 4, and 24 h after transplantation respectively, aP<0.05 vs control group).
Malondialdehye (MDA) level
As shown in Figure 3, the lipid peroxidation derived MDA level at 1.5 and 4 h after transplantation was significantly suppressed by UTI treatment (7.93±0.32 vs 10.97±0.77, 7.70±0.44 vs 9.70±0.77 mmol/g, n = 6, P<0.05). At the following later time points (24 and 72 h after transplantation), however, the graft MDA in both groups reverted to a similarly lower level.
Figure 3.
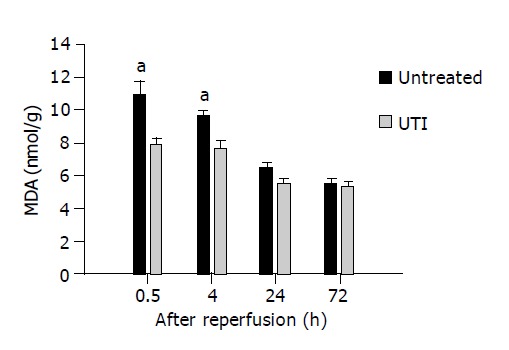
The MDA level at 1.5 and 4 h after transplantation was significantly suppressed in UTI-treated group, aP<0.05 vs the untreated control group.
Graft infiltration by CD11b+ Gr1+ monocytes
As shown in Figure 4, when the monocyte gate of graft lamina propria was analyzed at the third post-transplant day, the sub-population of CD11b+ Gr1+ cells were found reduced in the UTI-treated group. The percentage of CD11b+ Gr1+ cells, mainly neutrophils and macrophages, in untreated allografts was 10.3±0.7%, and was decreased to 1.0±0.2% in the UTI-treated group (P<0.01). It indicated less inflammatory infiltrations, particularly neutrophils and macrophages, into the intestinal graft by UTI therapy.
Figure 4.
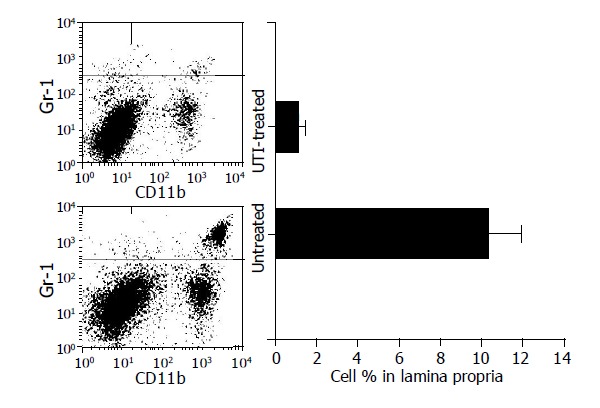
The sub-population of CD11b+ Gr1+ cells in the monocyte gate of graft lamina propria was found reduced in the UTI-treated group on the third post-transplant day.
Expression of i-NOS, TNFα mRNA in intestinal grafts
In the sham-operated mice, the expression of i-NOS and TNFα mRNA in non-ischemic intestine is minor. However, the expression of the intestinal graft elevated dramatically due to I/R events after transplantation. The expression of i-NOS mRNA reached 2.18±0.28 to 2.73±0.28 (TNFα/β-actin, n = 6), and the TNFα mRNA were 1.71±0.24 to 2.67±0.71 (i-NOS/β-actin, n = 6) within the first 72 h after transplantation. However, in UTI group, the expression of i-NOS mRNA was apparently inhibited in each time point, and the level of TNFα mRNA was also lower than the control group at 1.5 and 4 h after transplantation. It indicated a profound suppression of i-NOS and TNFα expression by UTI treatment, when challenged by transplant-associated I/R. Figures 5, 6 displayed i-NOS and TNFα mRNA expression at different time points after transplantation and representative images.
Figure 5.
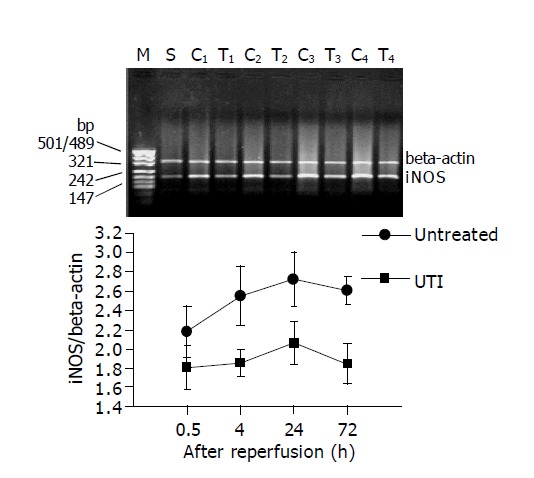
In UTI group, the expression of i-NOS mRNA was apparently inhibited at 1.5, 4, 24, and 72 h after intestinal transplantation. The upper bands show the representative images of the PT-PCR results for i-NOS. M: band for molecular marker; S: Sham operation group; C1-C4: bands for untreated group at 1.5, 4, 24 and 72 h after transplantation respectively; T1-T4: bands for UTI-treated group at 1.5, 4, 24 and 72 h after transplantation, respectively.
Figure 6.
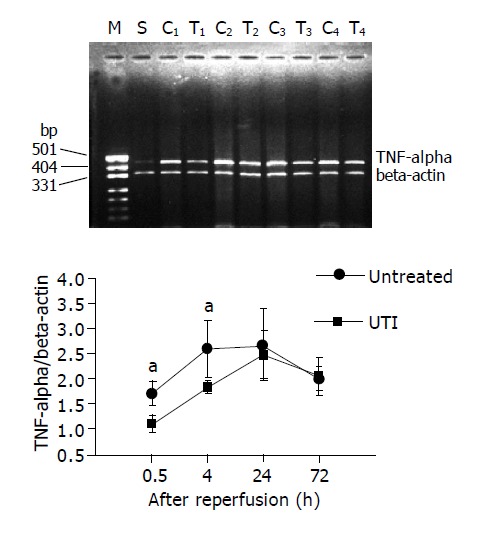
The expression of TNFα mRNA was inhibited at 1.5 and 4 h after intestinal transplantation in UTI-treated group, while no difference was found at 24 and 72 h after transplantation. The upper bands show the representative images of the PT-PCR results for TNFα. M: band for molecular marker; S: Sham operation group; C1-C4: bands for untreated group at 1.5, 4, 24 and 72 h after transplantation respectively; T1-T4: bands for UTI-treated group at 1.5, 4, 24 and 72 h after transplantation, respectively.
DISCUSSION
Intestine is one of the most vulnerable organs to I/R injury in solid organ transplantation[3]. The high morbidity and mortality after intestinal transplantation are unexceptionally associated to graft impairments due to I/R injury. Therefore, effective measures with minimal risk of evoking allogeneic rejection should be developed for mitigating the intestinal impairments caused by I/R response[9].
The most common model for investigations of intestinal I/R is performed by temporary occlusion of superior mesenteric artery (SMA)[10]. Easy as it is, however, it is unable to feature the I/R events occurring in intestinal transplantation. First, I/R injury, in transplantation, is mainly due to a longer cold ischemic preservation, while the SMA occlusion model can merely mimic a shorter warm ischemia. Second, collateral vascular supply cannot be excluded by SMA occlusion, which usually appears as sub-mucosal hemorrhage that is absent in transplantation. Other factors affecting the investigating outcomes, such as irrigation of the graft lumen during organ procurement, cannot be imitated by SMA occlusion either[3]. Thus, we took the more complicated mouse transplantation model to estimate the effect of UTI, in order to avoid possible errors.
Constitutive NO produced by c-NOS is a well-known mediator in several physiological processes. When insulted by inflammatory stimuli such as ischemia, i-NOS expression is promoted and a larger amount of NO is produced by i-NOS catalysis. Instead of being a signal transductor in lower levels, the highly concentrated NO acts as free radicals and eventually leads to local tissue damage[11]. Therefore, over expression of i-NOS is the key point for the NO mediated inflammatory reaction. TNFα is another crucial mediator in the onset and sustaining of the I/R response in the gut [12]. The present study reveals a profound inhibitive effect of UIT toward the expression of i-NOS mRNA in intestinal graft, which was challenged by long-term cold ischemia and followed reperfusion. The decreased levels of i-NOS mRNA and TNFα mRNA in the grafts are well consistent with the attenuated villous injury, as well as the reduced neutrophil index (MPO) and improved membrane stability (MDA). It suggested that the inhibition of TNFα and i-NOS, thereby, breaking down the cytokine cascade and the NO-induced tissue injury could be the underlying mechanism of the protective effects of UTI in intestinal transplantation.
Macrophages and other monocytes are the major source of TNFα and i-NOS mRNA,[13] therefore we isolated these infiltrated cells from the intestinal grafts and measured them by flow cytometry. Gr1 is usually expressed on granulocytes and macrophages, and CD11b has the similar tendency while it also appears on activated and mature lymphocytes[14]. The subpopulation, CD11b+ Gr1+ cells, including most neutrophils and macrophages, were found markedly reduced in UTI-treated grafts when compared to untreated grafts at the third post-transplant day. Hence, the lower level of TNFα and i-NOS mRNA in UIT-treated group might owe to reduced infiltration of macrophages and neutrophils. On the other hand, the suppressive effect of UTI on TNFα and i-NOS expression, if there is any, may lessen the severity of graft infiltration by abating the cytokine or chemotactic response. Shortly, prevention of macrophages and other monocytes into the graft by UTI is a possible mechanism for its alleviating effects on I/R injury.
As a safe and well-tolerated agent, UTI has not found any rejection-related adverse effects in clinical transplantation[15]. The revealed mechanism of UTI as well as our findings, such as inhibition of TNFα and i-NOS production, theoretically excludes the potential risk of provoking alloimmune response[16]. Consequently, UTI could be an ideal agent in protection of intestinal graft from IR injury. Further investigations for UTI in other solid organ transplants and clinical trials are required.
Footnotes
Supported by the Health Scientific Grant 2002 of Zhejiang Province, China. No. 2002ZX021
Science Editor Li WZ Language Editor Elsevier HK
References
- 1.Platell CF, Coster J, McCauley RD, Hall JC. The management of patients with the short bowel syndrome. World J Gastroenterol. 2002;8:13–20. doi: 10.3748/wjg.v8.i1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fishbein TM, Gondolesi GE, Kaufman SS. Intestinal transplantation for gut failure. Gastroenterology. 2003;124:1615–1628. doi: 10.1016/s0016-5085(03)00375-5. [DOI] [PubMed] [Google Scholar]
- 3.Newell KA, Fishbein TM. Experimental models of small bowel transplantation. Curr Opin Organ Transplant. 2003;8:209–216. [Google Scholar]
- 4.Kobayashi H, Gotoh J, Fujie M, Terao T. Characterization of the cellular binding site for the urinary trypsin inhibitor. J Biol Chem. 1994;269:20642–20647. [PubMed] [Google Scholar]
- 5.Yamaguchi Y, Ohshiro H, Nagao Y, Odawara K, Okabe K, Hidaka H, Ishihara K, Uchino S, Furuhashi T, Yamada S, et al. Urinary trypsin inhibitor reduces C-X-C chemokine production in rat liver ischemia/reperfusion. J Surg Res. 2000;94:107–115. doi: 10.1006/jsre.2000.5999. [DOI] [PubMed] [Google Scholar]
- 6.Li XK, Suzuki H, Kimura T, Kawabe A, Uno T, Harada Y. Ulinastatin, a protease inhibitor, attenuates intestinal ischemia/reperfusion injury. Transplant Proc. 1994;26:2423–2425. [PubMed] [Google Scholar]
- 7.Nakahama H, Obata K, Sugita M. Ulinastatin ameliorates acute ischemic renal injury in rats. Ren Fail. 1996;18:893–898. doi: 10.3109/08860229609047715. [DOI] [PubMed] [Google Scholar]
- 8.Zhong R, Zhang Z, Quan D, Garcia B, Duff J, Stiller C, Grant D. Intestinal transplantation in the mouse. Transplantation. 1993;56:1034–1037. [PubMed] [Google Scholar]
- 9.Newell KA. Transplantation of the intestine: is it truly different? Am J Transplant. 2003;3:1–2. doi: 10.1034/j.1600-6143.2003.30101.x. [DOI] [PubMed] [Google Scholar]
- 10.Pillai SB, Luquette MH, Nowicki PT, Besner GE. Segmental intestinal ischemia: an improved method of producing small bowel injury. J Invest Surg. 1998;11:123–128. doi: 10.3109/08941939809032191. [DOI] [PubMed] [Google Scholar]
- 11.Hassoun HT, Weisbrodt NW, Mercer DW, Kozar RA, Moody FG, Moore FA. Inducible nitric oxide synthase mediates gut ischemia/reperfusion-induced ileus only after severe insults. J Surg Res. 2001;97:150–154. doi: 10.1006/jsre.2001.6140. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto S, Tanabe M, Wakabayashi G, Shimazu M, Matsumoto K, Kitajima M. The role of tumor necrosis factor-alpha and interleukin-1beta in ischemia-reperfusion injury of the rat small intestine. J Surg Res. 2001;99:134–141. doi: 10.1006/jsre.2001.6106. [DOI] [PubMed] [Google Scholar]
- 13.Kim JY, Jeong HG. Down-regulation of inducible nitric oxide synthase and tumor necrosis factor-alpha expression by bisphenol A via nuclear factor-kappaB inactivation in macrophages. Cancer Lett. 2003;196:69–76. doi: 10.1016/s0304-3835(03)00219-2. [DOI] [PubMed] [Google Scholar]
- 14.William E, Paul M. 5th edition. Lippincott Williams & Wilkins Publishers, 2003. Fundamental Immunology. [Google Scholar]
- 15.Watanabe T, Sato Y, Ichida T, Yamamoto S, Oya H, Nakatsuka H, Kobayashi T, Hatakeyama K. Comparison of urinary ulinastatin levels between donors and recipients immediately following adult living related donor liver transplantation. Transplant Proc. 2003;35:76–77. doi: 10.1016/s0041-1345(02)03990-8. [DOI] [PubMed] [Google Scholar]
- 16.Itabashi K, Ito Y, Takahashi T, Ishii K, Sato K, Kakita A. Protective effects of urinary trypsin inhibitor (UTI) on hepatic microvasculature in hypotensive brain-dead rats. Eur Surg Res. 2002;34:330–338. doi: 10.1159/000063074. [DOI] [PubMed] [Google Scholar]


