Abstract
Sweet taste receptors are transmembrane protein network specialized in the transmission of information from special “sweet” molecules into the intracellular domain. These receptors can sense the taste of a range of molecules and transmit the information downstream to several acceptors, modulate cell specific functions and metabolism, and mediate cell-to-cell coupling through paracrine mechanism. Recent reports indicate that sweet taste receptors are widely distributed in the body and serves specific function relative to their localization. Due to their pleiotropic signaling properties and multisubstrate ligand affinity, sweet taste receptors are able to cooperatively bind multiple substances and mediate signaling by other receptors. Based on increasing evidence about the role of these receptors in the initiation and control of absorption and metabolism, and the pivotal role of metabolic (glucose) regulation in the central nervous system functioning, we propose a possible implication of sweet taste receptor signaling in modulating cognitive functioning.
1. Introduction
Taste receptors are integral plasma membrane proteins that recognize sapid substances, code information received from these substances, and transmit the information into intracellular acceptors. Taste receptors are divided into two types: type 1 receptor recognizes sweet molecules (see examples below); type 2 recognizes bitter molecules such as toxins, acids, and alkaloids. Both receptor types were only recently characterized [1, 2] and are increasingly studied in recent time. Type 1 receptor is further subdivided into three subtypes (T1R1, T1R2, and T1R3). For type 2 receptor, at least 25 subtypes are known to exist in humans [1, 3, 4].
This paper deals only with the signaling network of the sweet taste receptors, precisely the role of their signaling network in cognitive functioning. Sweet taste receptor signaling network is a complex communication pattern involving the regulated signaling of sweet molecules activating downstream target of taste cells and resulting in the perception of taste as well as modulation of related signaling pathways. The network involves the activating substrate, sweet taste receptor, intracellular molecules and cooperatively associated receptors, secretory peptides, molecules, and ions. It is suggested that through these components, sweet taste receptors modulate paracrine signaling pathways and can significantly affect neighboring cells by changes in ion (calcium) waves and activity-dependent signaling.
The activating ligands of sweet taste receptor are diverse and include both artificial (acesulfame potassium, aspartame, neotame, sucralose saccharin, or glycyrrhizin) and natural (glucose, lactose, fructose, galactose, maltose, and sucrose; amino acids including glycine, alanine, threonine, D-tryptophan, and D-histidine; the dipeptide L-aspartyl-L-phenylalanine and sweet proteins such as monellin, thaumatin, and brazzein) sweet substances [5–7]. Functional forms of the sweet taste receptor subtypes are known to exist in dimers. For instance, T1R2 forms a dimer with T1R3 (T1R2+T1R3 heterodimer). Formation of dimers and complexes allows the sweet taste receptors to detect various types of taste [4].
Sweet taste receptors have multisystem localization. The existence of sweet taste receptors was initially proposed by Newson et al. (1982) and later discovered in the gastrointestinal tract [8, 9] and then in the pancreas [7, 10–15]. They are also present in macrophages [16] and respiratory track, where it is believed to play significant role in the maintenance of the mucosal and ciliary functioning, in part, by ensuring adequate and supportive role for the sensing of tasty substances, as well as the clearance of glucose through GLUT 1 and GLUT 10 receptor types present in the respiratory track [17]. The supportive role of sweet taste receptors to glucose absorption and metabolism is proposed to play a part in the gastrointestinal tract [18], and this role probably is mediated through paracrine signaling or cross-talks [19]. These receptors are known to play a vital role in the initiation and progression of pathological process in the respiratory track (inflammation, asthma, etc.), gastrointestinal tract, and pancreas (metabolic disease such as diabetes) [7, 14, 15].
Interestingly, sweet taste receptors have been discovered in the visual, auditory, and olfactory systems, where they are known to modulate taste through visual, auditory, and olfactory perception, respectively [20–23].
Researchers have shown that sweet taste receptors are also located in the central nervous system (CNS), precisely in the hypothalamus. Ren and colleagues [24] showed that sweet taste receptor T1R2+T1R3 heterodimer is responsible for sensing glucose in the hypothalamus. This discovery could have implication for a better understanding of brain functioning and could provide information on mechanism of CNS disorders in which dysregulation of metabolism (glucose) is observed [25, 26].
The continuous search for different treatment options of cognitive disorders or the prevention of such conditions provides a substantial argument for constantly rising prevalence of CNS disorders in the world. Over the past decades, there has been constant increase in the prevalence of CNS disorders. It has been estimated that the number of people suffering from CNS disorders will get a whooping increase by 2020. Millions of people are mentally disabled, with the highest proportion occurring in ages 10–29 years [25]. Only between 1990 and 2010, the burden of disorders associated with CNS increased by 37.6%. In 2011, it was reported that neuropsychiatric disorders (accounting for 45%) were the leading cause of disability for people aged 10–24 years [25, 27]. These data suggest that, indeed, there is increasing necessity to search for new frontiers in both metabolic and cognitive functioning of the CNS. This is based on increasing evidences suggesting that metabolic disorders precede cognitive dysfunction [28]. Cerebral metabolic regulation is key to normal cognitive functioning and might in fact be a key predictor of cognitive functioning and diseases related to brain functioning. In fact, in an analysis, it was observed that cerebral glucose regulation parameter for the identification of cognitive dysfunction was more effective and efficient than the neuropsychological tests that are used for the diagnosis of cognitive impairment [28]. For instance, researchers have shown that the initial stage of Alzheimer's disease involves decrease in brain glucose metabolism by 45%, whereas blood flow decrease by only ~18% [29]. Assuming conservatively that the prevalence of the disease remains constant, it is estimated that for Alzheimer's disease alone, compared with the prevalence as at 2010, the number of cases will double, hitting 65.7 million by 2030 and 115.4 by 2050 [30]. It is estimated that the sharpest increases in the disease are expected to hit low and middle income countries [31]. A huge amount of economic, societal, and psychosocial costs accrue from cognitive impairments [32–34]. In 2010, the total worldwide cost of cognitive impairment was estimated at US$604 billion [31]. In the US alone, an estimated cost of caregiving in 2012 was at $216 billion [35], suggesting a sharp increase in the economic cost due to the increase in the prevalence of the disease. A total of $536 billion and $1.75 trillion are minimum estimates of the long-term losses to the US economy in 1991 caused by Alzheimer's disease [36]. Without doubt, it is obvious that understanding how the sweet taste receptors could affect metabolic and cognitive functions of both neurons and astrocytes could provide plausible information on treatment option of some CNS disorders that have recorded tremendous increase in rent times. Of paramount importance is how efficient hypothalamic metabolic regulation relates to the regulation of metabolism in other parts of the brain (such as the hippocampus and cortex).
Based on data that suggest possible role of the sweet taste receptors in controlling sugar absorption and metabolism and recent reports that metabolic disorders precede cognitive impairment, it is suggested in this work that the activity of this sweet taste receptor-signaling network could have implication for the regulation of some aspects of cognitive functioning. In this paper, recent studies that suggest a possible role of the sweet taste receptor in the neural control of metabolism and cognition are reviewed. The possible mechanisms linking the cognitive and metabolic functions of the sweet taste receptors are also proposed.
2. General Model of Signaling of Sweet Taste Receptors
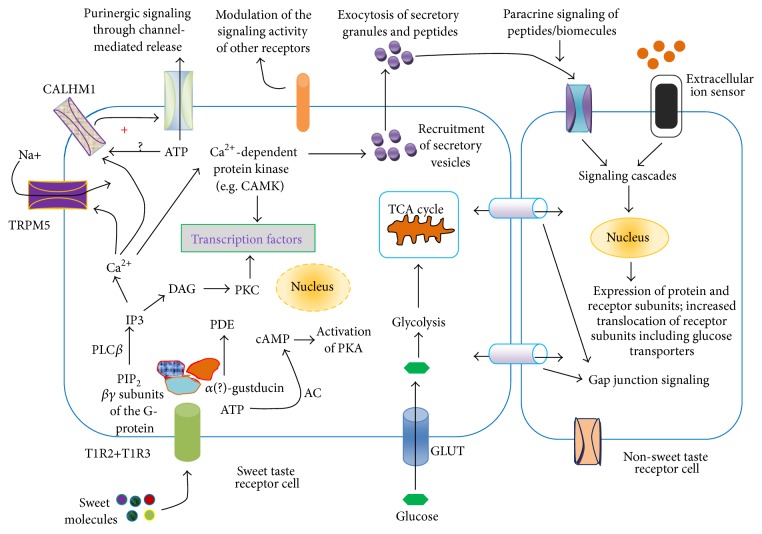
It is at least 3 decades since the initial hypothesis about taste receptors was made in early 1980s by Newson and colleagues [37]. However, experimental results on the presence of these receptors showed up in the literature only after a decade following the proposal of Newson and colleagues [8, 9]. Literature data point to the extensive development of sweet taste receptor signaling in the last half-decade. This development involved not only the unraveling of some of the mechanisms of sweet taste receptor signaling but also the diversity in the localization. The discovery of sweet taste receptors in the brain is a key to better understanding of certain aspect of brain functioning. Ren et al. [24] reported the localization of sweet taste receptors in the brain and suggested that these receptors serve as glucosensor in the hypothalamus. Signaling mechanisms of sweet taste receptors in the identified tissues and cells bear some similarities. In this next section, a general model of sweet taste receptor signaling will be outlined; thereafter, the role and mechanisms of these receptors in metabolic and cognitive functions shall be discussed. The general concept of sweet taste receptor signaling is shown in Figure 1.
Figure 1.
A general model of sweet taste signaling network. Sweet taste receptors possess multiple binding sites and mode of interaction for sweet molecules and they belong to class C of heterotrimeric guanine nucleotide-binding protein, G-protein [143–145]. Sweet molecules activate the G-protein by downstream signaling leading to the dissociation of the α-subunit gustducin from the βγ subunits [146, 147]. Dissociated βγ subunits of the G-protein activate phospholipase Cβ (PLCβ), leading to the formation of 1,4,5-inositol trisphosphate (IP3). IP3 is responsible for the release of Ca2+ from intracellular stores through its binding to IP3-receptor in these stores. Increase in intracellular Ca2+ activates calcium dependent kinase, monovalent selective cation channel, TRPM5 (transient receptor potential cation channel, subfamily M, member 5) [15, 44, 146], and other receptors [44, 148]. To establish the role of TRPM5 or PLCβ (type 2), Zhang et al. [4] showed that knockout of the receptor or the enzyme abolishes the sensation of taste in cells. TRPM5 may also play a role in capacitance mediated calcium entry into taste cells [147]. Modulation of purinergic signaling by taste receptor also plays useful role in taste sensation. For the initiation of purinergic release, it was recently demonstrated by Taruno et al. [148] that the voltage-gated ion channel, calcium homeostasis modulator 1 (CALHM1), is indispensable for taste-stimuli-evoked ATP release from sweet, bitter, and umami taste cells. Importantly, CALHM1 is expressed not only in sweet but also in bitter and umami taste sensing type 2 cells. Taruno et al. [148] proposed that CALHM1 is a voltage-gated ATP-release channel. Dissociated α subunit referred to as Gα-gustducin activates a phosphodiesterase (PDE) thereby decreasing intracellular cAMP levels [146, 149]. Gα-gustducin is also reported to activate adenylate cyclase (AC) to increase cAMP level [150]. According to earlier report, Clapp et al. [151] demonstrated that, compared to wild type mice, knockout of α-gustducin in the taste buds of mice resulted in high basal (unstimulated) cAMP level. The results of these authors [151] indicated that α-gustducin is necessary to maintain low level of cAMP level. Low level of cAMP is necessary to maintain the adequate signaling of Ca2+ by disinhibition of cyclic nucleotide-inhibited channels to elevate intracellular Ca2+ [38]. Changes in cAMP levels also affect other ion channels, including K+ channels. The events resulting in activation/modulation of ion channels lead to membrane depolarization and formation of action potentials. Potential-dependent release of mediators (ATP, serotonin, etc.) and peptides and calcium dependent release of peptides/biomolecules are some of the results of sweet taste receptor signaling [152]. A hallmark of sweet taste receptor signaling is the activation of transcription factors and gene expression, which might be dependent on calcium and activity dependent activation calcium dependent kinases, including the calmodulin-dependent protein kinase (CAMK). Activation of protein kinases may be achieved through other signaling pathways. It appears that sweet taste receptor signaling involves multiple activating substrates and different types and subtypes of both α-gustducin and βγ subunits of the G-protein. Although, different subtypes of sweet taste G-protein receptor subunits have been known for over a decade, their specific roles in sensing taste are not exactly clear [38, 149, 153]. For instance, Huangu et al. [149] reported the presence of β1 and γ13. The sweet taste receptor is also known to have β3 subtype subunit. For α-gustducin, Gα i-2, Gα i-3, Gα 14, Gα 15, Gα q, Gα s, α-transducin have been identified [38].
The model (Figure 1) shows the mechanism of signaling initiated by sweet substances in sweet taste cell and possible effect on neighboring nonsweet taste cell (through paracrine or gap junction communication). One aspect of research into sweet taste receptor signaling that is yet to be reported is how different concentrations of sweet substances affect signaling.
The signaling events (Figure 1) lead to the modulation of cellular activities, propagation of action potential, and the paracrine signaling [38–40], including the release of peptides (glucagon-like peptide-1, GLP-1) and several biomolecules [41]. The functions of GLP-1 are well documented in the brain and gastrointestinal system [42].
Increasing evidences suggest that sweet taste signaling mechanisms in the gastrointestinal tract are similar to those in the pancreas and respiratory tract [19]. Nakagawa et al. [43] showed that sweet taste receptors of the pancreatic β-cell (by the addition of sucralose) activate the calcium and cAMP signaling systems to stimulate insulin secretion. They demonstrated that the secretory activity of the second pool of insulin vesicles blocked by the dihydropyridine L-type calcium channel blocker, 3,5-dimethyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate (nifedipine). In addition, the IP3-receptor inhibitor, 2-aminoethoxydiphenyl borate, effectively blocked Ca2+ waves caused by both the first and the second pool of insulin secretion. More so, the peptide, gurmarin, an inhibitor of the sweet taste receptor, blocked calcium response to the artificial sweetener, sucralose [43]. Gurmarin can inhibit sweet taste responses 30–80% to multiple sweet molecules, including glucose, sucrose, saccharin, and SC45647; however, it has no inhibitory effect on salty, sour, or bitter compounds. Another known inhibitor of sweet taste receptor includes the proteolytic enzyme pronase [44]. These results are in agreement with the reports of [45] and data reported elsewhere [46] for other tissues and cells. The heterodimer sweet taste receptor, T1R2/T1R3, in the presence of amiloride (3 mM) loses its response to sweet taste molecules such as sucrose, artificial sweetener, and sweet protein. Another sweet taste inhibitor, lactisole, is known to inhibit this response but maybe these inhibitors may possess different binding sites on the sweet taste receptor dimers [45].
The presence of multiple sweet taste signaling pathways is based on experiments suggesting that gurmarin inhibits some but not all sweet taste responses of the chorda tympani nerve to sweet molecules in experimental animal [44]. Investigators showed that gurmarin inhibition of sweet taste receptors was dependent on many factors, including temperature, suggesting that multiple factors, including peptides, environmental factors, and higher brain functions (such as emotion) may play a role in modulating taste perception. Yoshida et al. [47] recently demonstrated that the anorexigenic mediator and adipocyte hormone, leptin selectively suppresses sweet taste sensitivity via the adiposity receptor, Ob-Rb, in sweet taste cells. Whereas orexigenic mediators, endocannabinoids, notably, anandamide [N-arachidonoylethanolamine], and 2-arachidonoyl glycerol, selectively enhance sweet taste via cannabinoid receptor type 1 in sweet taste cells. These mediators act centrally in the hypothalamus and limbic forebrain [47]. While this present work did not find any study investigating the effect of insulin on sweet taste receptors, it could be expected that increase in insulin secretion signaling will lead to suppression of sweet taste, while glucagon will enhance sweet taste perception in sweet taste cells.
3. The Sweet Taste Receptor Heterodimer, T1R2+T1R3, as a Model Sensor of Glucose in the Neuroastroglial System: Window of Interaction with Cognitive Control Systems
Generally, sensors of glucose could be divided into plasma membrane glucosensors and intracellular glucosensors. The functions of these different types of glucosensors will be discussed in more details in another paper under preparation. In this section, only the plasma membrane glucosensor, T1R2+T1R3, is discussed, precisely, as a hypothalamic glucosensor serving to modulate metabolic and cognitive functions in the neuroastroglial system. However, it is important to note that there are numerous interactions between the plasma membrane and intracellular glucosensors, which will be briefly outlined in course of our discussion. At present, in the world literature, known plasma membrane receptors that serve as glucosensors are the second member of the glucose transporter (GLUT2) and the third member of sodium/glucose cotransporter, SGLT3. The heterodimer, T1R2+T1R3, is a fairly new member of the plasma membrane glucosensors.
As earlier noted, numerous sweet molecules can activate the T1R2+T1R3; however, because our discussion in this section is directed to the neuroastroglial system, we shall limit the ligand of sweet taste receptor to glucose molecule alone. This is because glucose is the chief metabolic substrate for the brain. The daily requirement of glucose for the brain of an adult is about 120 g of the total amount of 160 g of glucose needed daily for the whole organism [46, 48]. During brain activation (as in mental activities), glucose uptake by brain cells can be increased up to ~90%. Our previous analysis also showed that the contribution of blood glucose level necessary to maintain the function of the brain is about 40% and may increase to 90% or more during prolonged mental work [49–51]. During hypoglycemia, brain function is greatly reduced, but ketone bodies serve (especially during continuing and prolonged fasting) to provide a significant part of the energy needs of the brain [52, 53]. However, it should be noted that ketone bodies are not able to maintain or restore the normal function of the brain in the absence of glucose [46]. In our previous analysis, decrease in glucose level was associated with statistically significant decrease in brain functions [54]. The lowering of brain functions following decrease in blood glucose level, accompanied by a corresponding decrease in cerebral glucose level is reported by McNay and Sherwin [55] and reviewed by McNay and Cotero [56] and also documented elsewhere [57–59].
The mechanisms involved in the role of sweet taste signaling [24, 60–63] in cognitive functions may largely involve signaling through metabolic coupling; activity dependent signaling; cross-signaling, initiated by downstream effectors; and receptor cooperativity and associativity. These processes may be mediated through paracrine signaling, activities of extracellular ion sensors, and ion homeostasis, which in turn may modulate the functions of the neuroastroglial system.
Ren et al. [24] found sweet taste receptors (T1R1,2,3 and their heterodimers: T1R2/T1R3 and T1R1/T1R3) as well as their corresponding G-protein genes in the neurons of the nuclei paraventricularis and arcuatus of the hypothalamus, CA area and dentate gyrus of the hippocampus, habenula, cortex, and intraventricular epithelial cells of the choroid plexus. Interestingly, the highest level of taste-related G-protein gene expression was found in the hypothalamus, followed by cortex and hippocampus [24]. The hypothalamus has been long known to serve as a site of regulation of feeding, central and peripheral metabolism, hormones secretion, and functions [24]. Expression of these receptors is associated with a physiological condition (fasting increases the amount of T1R1 and T1R2 decrease as hypothalamic glucose leads to an increase in the expression of T1R1, T1R2, which normalizes when sweet molecules were administered). Moreover, these brain areas identified to express taste receptor genes are implicated in cognitive functioning [64–66]. It is possible that receptor cooperativity or associativity with the sweet taste receptor could as well affect signaling in other pathways [67].
The chief signaling relationship between the activities of plasma membrane glucose sensor (which is also linked to intracellular energy sensors) and cognitive control systems is made possible, majorly, through metabolic coupling in the neuroastroglial system [61]. Importantly, metabolic coupling could have profound effect on activity dependent signaling; cross-signaling, initiated by downstream effectors; and receptor cooperativity and associativity by majorly modulating paracrine signaling, activities of extracellular ion sensors, and ion homeostasis [18, 24, 67].
3.1. Signaling through Metabolic Coupling: A Window of Interaction between Cognition and Metabolic Functions
The activity of the neural plasma membrane glucosensor, T1R2+T1R3, from the work of Ren et al. [24] is evident that the heterodimer largely contributes to maintaining the activity of the cell by controlling glucose transport. This is possibly achieved by the associativity and/or cooperativity between the GLUT2 and T1R2+T1R3. How the T1R2+T1R3 receptor affects the glucose transporters is not known, but it may be suggested that the signaling mechanisms might involve downstream effectors that subsequently modulate both the transport activity and the expression of the GLUT2 sensor. In the gastrointestinal tract, for instance, T1R2+T1R3 receptor has been shown to influence the activity and expression of GLUT2 and SGLT1, possibly through autoparacrine signaling [18]. Moreover, Margolskee et al. [18] reported that this receptor heterodimer controls glucose absorption and metabolism.
Decrease in metabolic functions of the neurons results in decrease in neuronal activity. Thus, collateral neuronal and glial cell functions might also be affected. Shunts controlled by metabolic coupling such as the GABA/glutamate-glutamine cycle will also be affected since glucose serves as precursor [68–72]. Several neurotransmitter systems implicated in cognitive functioning are regulated by glucose or its metabolites [73, 74]. Analysis of the literature indicates a significant role of dopamine, glutamate, serotonin, cholinergic, and noradrenergic systems in cognitive functioning [61, 75]. Glucose is required for the synthesis of neurotransmitters such as serotonin, noradrenaline, and acetylcholine, which can affect both local and distant neural and astroglial population [61]. The neurotransmitters, ATP, d-serine, which may be synthesized from glucose, affect long-term potentiation (LTP), synaptic plasticity, information storage, and retrieval [75, 76]. Substances released from the neuroastroglial system can also initiate signaling in many pathways necessary for memory/cognitive functions [75, 76].
The release of these molecules could modulate not only cognitive functions but also different aspects of behavior, including emotion [75–77]. The functional link between the hypothalamus and brain areas of emotion is an indication of the possible influence of hypothalamus on cognitive functioning. Moreover, the hypothalamus itself is implicated in cognitive functioning as disorders involving the hypothalamus evidently result in cognitive dysfunction [78–83] (briefly discussed in the next section).
Downstream effects of T1R2+T1R3 might also involve energy sensors. The discovery of the presence of functional sweet taste receptors in the brain has thrown more light to our understanding of metabolic functioning involving glucose regulation. Metabolic or energy sensors in the neuroastroglial system include glucokinase, GLUT2 [84, 85], AMPK (AMP activated protein kinase), CREB (cAMP related element binding protein), mTOR (mammalian target of rapamycin), sirtuins, and PASK (or PASKIN and PSK, a kinase protein) [64, 86–90]. The study by Ren et al. [24] has shown that the sweet taste receptor heterodimer T1R2+T1R3 might be responsible for mediating the energy sensing activity of previously identified metabolic sensors. Although in Ren et al. [24] study the activities of other metabolic sensors were not put into consideration, knockout of sweet taste receptors may have considerable impact on intracellular energy sensors in the neuroastroglial network. However, it will be expected that decrease in glucose entry into the cell caused by disorder in sweet taste receptor signaling will actually mobilize intracellular substrates to counterbalance the change. Notwithstanding, this change will result in decrease in neural functions due to lowering of glucose entry into the cell.
There could be a functional association between the identified metabolic sensors and plasma membrane glucosensors. Recent study by Hurtado-Carneiro et al. [91] has shown that PASK that functions as a nutrient and energy sensor in hypothalamus is required for the normal functioning of other metabolic sensors, including AMPK and mTOR/S6K1. This functional relationship (or cross-talk) between metabolic sensors is useful in maintaining not only metabolic functions but also normal/adequate cognition. In this regard, previous data have consistently shown that AMPK, CREB, and mTOR are involved in both glucose metabolism and memory functions (reviewed in [61]) (both AMPK and CREB are strongly involved in T1R2+T1R3 signaling). Recent work by Hurtado-Carneiro et al. [92] indicates that the peptide GLP-1 can attenuate the activity of AMPK and mTOR/S6 kinase induced by fluctuations in glucose levels in hypothalamic areas involved in feeding behaviour. The functional relationship between some of these signaling pathways was analyzed in a recent review [93]. Disorders involving the mTOR significantly affect cognition and is associated with neuropsychiatric symptoms, including intellectual disability, specific neuropsychological deficits, autism, other behavioral disorders, and epilepsy [94].
Although, information is scanty, available data suggest that other signaling pathways that link glucose metabolism to cognitive functioning might include extracellular kinases [95–97]. These identified pathways could activate LTP, thereby enhancing memory formation or retrieval [98, 99] and can also function through activity-dependent signaling as well as activation of transcription factors [3, 11, 100]. Decrease in glucose is associated with deficits in memory and learning (possibly due to a decrease in LTP) [101, 102].
In the gastrointestinal tract, for instance, endocannabinoid receptors are reported to be associated with the fatty acid receptor necessary for sweet taste perception [67]. In the nervous system, the literature search in this present study did not produce such result; it can, however, be proposed that such associativity is possible. In this vein, it is demonstrated that the effects of cannabinoids are associated with dysfunctions of rapamycin pathway and extracellular signal-regulated kinases, suggesting a relationship between the signaling pathways, and possibly receptor cooperativity or associativity [103]. In Wang and Zhuo's paper [104], it was noted that stimulation of group I metabotropic glutamate receptors initiated a wide variety of signaling pathways that regulate gene expression at both the translational and transcriptional levels and induce translation or transcription-dependent synaptic plastic changes in neurons. This wide range of signaling by metabotropic glutamate receptors can activate other receptors and thus initiating the activity of numerous transcription factors and gene expression.
Of important note is the downstream effect of neuroastroglial glucose metabolic disorder resulting in changes in calcium ion concentration. The result of this is a disorder in synapse-to-nucleus communication involving several kinases (including the mitogen-activating protein kinase, MAPK; CAMK), gene expression, and synaptic plasticity [105, 106]. These protein kinases are responsible for regulating the activities of transcription factors including CREB, CCAAT (cytosine-cytosine-adenosine-adenosine-thymidine)/enhancer-binding protein (C/EBP), Early growth response protein (Egr) also known as zinc finger protein 225 (Zif268) or nerve growth factor-induced protein A (NGFI-A), activator protein-1 (AP-1), nuclear factor κB (NF-κB), c-Fos, and Elk-1, c-Jun. These proteins (transcription factors and kinases) are well implicated in metabolism and cognition [104–110]. LTP is dependent on the activities of protein kinases. Also, nitric oxide, which is thought to be associated with glucose metabolism [111, 112], also contributes to LTP by downstream targets, stimulating guanylyl cyclase and cGMP-dependent protein kinase, which acts in parallel with PKA to increase phosphorylation of the transcription factor CREB [106]. Most notably, activity-dependent signaling of calcium is also shown to play a pivotal role in neural and synaptic plasticity and regulation of gene expression [106].
It has been proven again and again that memory formation and retrieval involve short-term signaling associated with activation of transcription factors controlling immediate early genes (early response genes) and long-term signaling to the nucleus for the formation of long-term memory [113–117]. However, the fact that microtubules of cells might play a pivotal role in memory has only been explored recently. In the work of Craddock et al. [118], a possible encoding of memory in the microtubule lattices mediated by the phosphorylation of type II CaMK was reported. Whether extended research into mechanisms of memory coding in the microtubule lattices will help scientists find the long-time searched neural code for memory is yet unknown. In the work of Eric Kandel, who shared the 2000 Nobel Prize in Physiology or Medicine with Arvid Carlsson and Paul Greengard, best known for his research on the physiological basis of memory storage in neurons, documented in his Nobel Lecture, published in the 2001 issue of the “Bioscience Reports,” there are compelling evidences for the role CAMK in memory [113]. In page 597 of Eric Kandel's account of the mechanisms of memory storage, CAMK was noted to play a role in LTP [113]. In Craddock et al. report [118], activity dependent flux of postsynaptic calcium activated the dodecameric holoenzyme containing two hexagonal sets of 6 kinase domains, hexagonal CaMK type II. One bit of information encoded equals one protein kinase domain. Information conveyed through activity dependent calcium waves are encoded by the phosphorylation as arrays of binary “bits.” Six phosphorylated bits are equal to bytes. Thus, thousands of bytes of information can be encoded in one microtubule [118]. A number of studies have implicated the role of microtubules [119–121] and CaMK signaling in both normal cognitive functioning and diseases [122, 123].
Recent work suggests the mechanism for the involvement of microtubule network in the formation of memory [124]. Wang and Zhuo [104] recently showed that microtubule protein is regulated by activity dependent processes. The mechanism of its regulation indicate that synaptic changes are associated with activation of the corresponding gene (more precisely called immediate early gene) and gene expression. The early responsegene c-fos is also involved in activity dependent signaling and it is dependent on CREB activity [104]. Also, microtubule network is previously known to interact with nuclear materials necessary for the formation of memory [125]. The turnover rate of microtubule also correlates well with time needed for short-term and long-term memory formation. It is obvious that there are millions of microtubule proteins to which protein kinase (e.g., CAMK) can interact with; however, the approximate number is not known precisely as there are numerous disagreements in the literature as to the number of microtubule proteins present in a given neuron [126, 127].
3.2. The Relationship between Cognition and Hypothalamic Metabolic Functions: The Search Continues
Cognition (from Latin cognotio, to know, learn) is a higher brain function and comprises attention, memory, judgement, reasoning, problem solving, planning, decision-making, and language. It is related to emotion and behavior. Cognition is a function of multiple brain regions, majorly involving the cortical Brodmann areas, insula, anterior cingulate, thalamus, mediotemporal (including hippocampus), mediofrontal and prefrontal cortices, basal ganglia, and cerebellum [64–66, 128–133]. The hypothalamus plays a role in some aspect of cognition, mostly emotion and behavior [134–136]. The mammillary nuclei and medial nuclei of the posterior hypothalamus are involved in some aspects of cognitive functioning. Medial part of the tuberal hypothalamus (tuberal hypothalamus is at the level of the tuber cinereum, which is usually divided into medial and lateral parts) containing the arcuate nucleus is involved in feeding and dopamine release. Dopamine can act locally and at distant point away from the site of release to modulate cognition [77].
How will disruption of sweet taste receptor signaling affect cognitive functions? Hypothalamic dysfunction is involved in epilepsy, a condition characterized by higher brain function impairment, including cognitive dysfunction [137]. In addition, the hormones and peptides secreted by the hypothalamus are involved in cognition. The secretions of the hypothalamus mediated by metabolic activities could extend to other brain areas including the hippocampus and cortex and are believed to modulate various behavioral parameters: learning and memory, as well as neuroprotection, reproduction, growth, and metabolism [138]. For instance, investigation involving the growth hormone has been shown to modulate synaptic plasticity, thereby altering cognition. Growth hormone replacement therapy attenuates cognitive impairment [139]. Apart from the growth hormone, other hormones of the hypothalamus have been implicated in cognitive functioning. Importantly these hormones of the hypothalamopituitary axis are known to act on other brain areas involved in cognitive functioning: hippocampus and cerebral cortex [78–83]. Some of these hormones can modulate taste perception [140]. These hypothalamopituitary hormones have been implicated in cognitive dysfunction, including dementia. In progressive neurodegenerative diseases involving cognitive impairment, as in Alzheimer's disease, recent evidences have pointed to the involvement of metabolic disorders as the most reliable indicator in comparison with the traditional neuropsychological tests [29, 141]. In Alzheimer's disease, alterations in hypothalamic aminergic cholinergic system is reported [141]. In Alzheimer's disease, dysfunction of the transport activity of glucose transporters have been implicated in the pathogenesis of the disease, and in metabolic disease, including diabetes (reviewed in Shah et al. [142]). These data suggest that hypothalamic metabolic alteration will affect cognitive functioning through multiple mechanisms.
4. Conclusion
Based on the recent finding presented in this work, the hypothalamic glucosensor, heterodimer sweet taste receptor, could serve as a key controller of glucose absorption and metabolism in the brain. This newly novel neural glucosensor will provide further opportunities for translational research aimed at identifying new therapeutic target agents to treat certain related metabolic dysfunctions of central origin and cognitive impairment, which almost in all cases coexist with neural metabolic dysfunction. Research into the polymorphic forms of the sweet taste receptors (T1R2 and T1R3), genetic variations of α-gustducin and their relationship to sweet sensitivity will provide useful information, including individual differences in taste identification. Investigation of the cognitive function implication of metabolic signaling functions of key brain areas involved in metabolism and cognition (hypothalamus, cortex, and hippocampus) and sweet taste receptors signaling will likely provide further information that might help in improved treatment options for cognitive impairment or provide possible cues to prevention of such conditions.
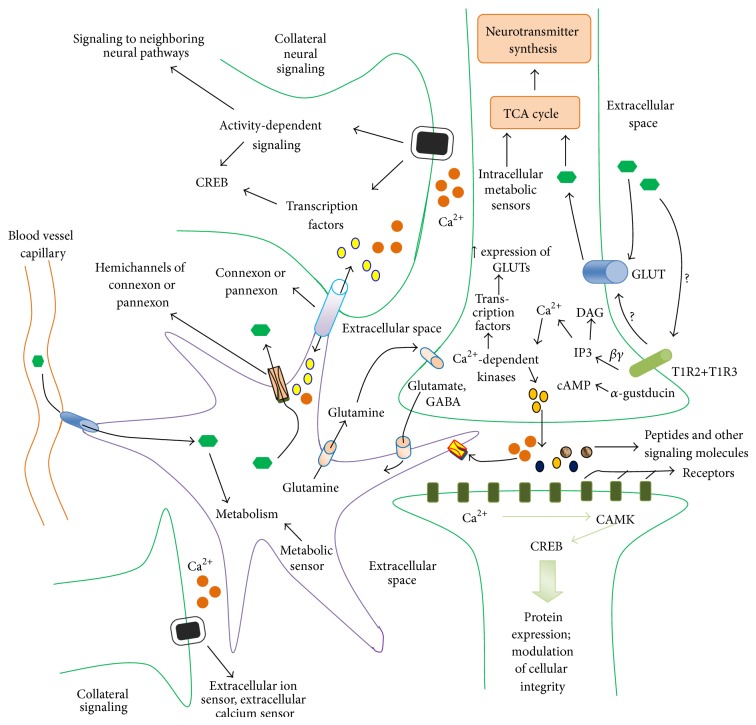
Figure 2.
Sweet taste signaling network of the neuroastroglial system. The brain is a complex network of cells, largely populated by neurons and astrocytes. There are ~100 billion neurons, with glial cells outnumbering neurons by about 10-fold. Astrocytes form the largest population of glial cells. The metabolic role of astrocytes in brain has been reviewed in our previous work [60]. Mechanisms of how glucose enters into the astrocytes and neurons are well documented [61]. From the scheme (Figure 2), the presynaptic neuron senses glucose mediated by the T1R2+T1R3 and GLUT2. While the mechanisms, by which the neuron senses glucose through GLUT2, have increasingly been defined, the glucosensing mechanisms of the sweet taste receptor are yet to be understood. It is quite possible that sweet taste receptor can modulate the plasma membrane GLUT2 glucosensor. Functioning cooperatively with GLUT2 to sense the metabolic rate of the intracellular milieu is the G-protein coupled receptor, the inwardly rectifying ATP-dependent potassium channel (KATP channel) [62, 63]. Transport activity of GLUT2 may be affected through multiple signaling pathways, such as those involving the regulation of GLUT2 and KATP channel activity. While in Ren et al. [24] study, the signaling activity of GLUT2 was not assessed, their results showed that the inhibition of sweet taste receptor resulted in increase in taste receptor gene expression, suggesting that sweet taste receptors persistently code information about the extracellular glucose level to intracellular milieu, and this might, probably, involve intracellular metabolic sensors, mediating neural activity, gene expression, and membrane receptor trafficking. Although, the mechanisms of the T1R2+T1R3/GLUT2-cooperativity/associativity (or intracellular metabolic sensors) interaction are not known, it can be proposed that T1R2+T1R3 could modulate GLUT2 transport activity through mechanisms as yet unknown. Mechanism of downstream signaling of the neuronal T1R2+T1R3 receptor is similar to that in other cells (Figure 1). The downstream signaling of these receptors can result in changes in extracellular calcium concentration as well as changes in peptide secretions. These biomolecules are sensed by their corresponding receptors in the adjacent neurons/astrocytes, which couple the received information into intracellular signal and cellular activity. The waves of calcium ions, peptide-dependent signaling, can affect collateral neurons and astrocytes by activity dependent signaling and changes in ion waves and regulate gene expression and protein synthesis. Several transcription factors and memory relation genes are activated/deactivated. Intercellular signaling through connexons and pannexons in these cells can modulate their activity.
Future Direction
Since sweet taste receptor heterodimer, T1R2+T1R3, is a novel glucosensor in the hypothalamus, further research in unraveling the signaling mechanisms associated with this receptor and other glucosensors in key brain areas is necessary to further understanding the association between glucosensors (including their relationship to metabolism) and cognitive functioning. In line with our earlier model of glucose memory facilitation [50, 61, 154], investigation into the mechanisms of neuroastroglial metabolic cooperativity in light of recent discoveries about sweet taste signaling is without doubt necessary.
Conflict of Interests
The authors would like to declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Nelson G., Hoon M. A., Chandrashekar J., Zhang Y., Ryba N. J. P., Zuker C. S. Mammalian sweet taste receptors. Cell. 2001;106(3):381–390. doi: 10.1016/S0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 2.Adler E., Hoon M. A., Mueller K. L., Chandrashekar J., Ryba N. J. P., Zuker C. S. A novel family of mammalian taste receptors. Cell. 2000;100(6):693–702. doi: 10.1016/S0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 3.Sainz E., Cavenagh M. M., LopezJimenez N. D., et al. The G-protein coupling properties of the human sweet and amino acid taste receptors. Developmental Neurobiology. 2007;67(7):948–959. doi: 10.1002/dneu.20403. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y., Hoon M. A., Chandrashekar J., et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112(3):293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 5.Blad C. C., Tang C., Offermanns S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nature Reviews Drug Discovery. 2012;11(8):603–619. doi: 10.1038/nrd3777. [DOI] [PubMed] [Google Scholar]
- 6.Pepino M. Y., Bourne C. Non-nutritive sweeteners, energy balance, and glucose homeostasis. Current Opinion in Clinical Nutrition & Metabolic Care. 2011;14(4):391–395. doi: 10.1097/mco.0b013e3283468e7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kojima I., Nakagawa Y. The role of the sweet taste receptor in enteroendocrine cells and pancreatic β-cells. Diabetes and Metabolism Journal. 2011;35(5):451–457. doi: 10.4093/dmj.2011.35.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Höfer D., Püschel B., Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of α-gustducin. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(13):6631–6634. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaughlin S. K., McKinnon P. J., Margolskee R. F. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357(6379):563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 10.Kim U., Wooding S., Ricci D., Jorde L. B., Drayna D. Worldwide haplotype diversity and coding sequence variation at human bitter taste receptor loci. Human Mutation. 2005;26(3):199–204. doi: 10.1002/humu.20203. [DOI] [PubMed] [Google Scholar]
- 11.Sbarbati A., Tizzano M., Merigo F., et al. Acyl homoserine lactones induce early response in the airway. Anatomical Record. 2009;292(3):439–448. doi: 10.1002/ar.20866. [DOI] [PubMed] [Google Scholar]
- 12.Tizzano M., Gulbransen B. D., Vandenbeuch A., et al. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(7):3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee R. J., Xiong G., Kofonow J. M., et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. Journal of Clinical Investigation. 2012;122(11):4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima I., Nakagawa Y., Ohtsu Y., Medina A., Nagasawa M. Sweet taste-sensing receptors expressed in pancreatic β-cells: sweet molecules act as biased agonists. Endocrinology and Metabolism. 2014;29(1):12–19. doi: 10.3803/enm.2014.29.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyriazis G. A., Soundarapandian M. M., Tyrberg B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(8):E524–E532. doi: 10.1073/pnas.1115183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taya K., Hirose K., Hamada S. Trehalose inhibits inflammatory cytokine production by protecting IκB-α reduction in mouse peritoneal macrophages. Archives of Oral Biology. 2009;54(8):749–756. doi: 10.1016/j.archoralbio.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Lee R. J., Kofonow J. M., Rosen P. L., et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. The Journal of Clinical Investigation. 2014;124(3):1393–1405. doi: 10.1172/jci72094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margolskee R. F., Dyer J., Kokrashvili Z., et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(38):15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Depoortere I. Taste receptors of the gut: emerging roles in health and disease. Gut. 2014;63(1):179–190. doi: 10.1136/gutjnl-2013-305112. [DOI] [PubMed] [Google Scholar]
- 20.Höfer D., Asan E., Drenckhahn D. Chemosensory perception in the gut. News in Physiological Sciences. 1999;14(1):18–23. doi: 10.1152/physiologyonline.1999.14.1.18. [DOI] [PubMed] [Google Scholar]
- 21.Braun T., Voland P., Kunz L., Prinz C., Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology. 2007;132(5):1890–1901. doi: 10.1053/j.gastro.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 22.Mace O. J., Lister N., Morgan E., et al. An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine. The Journal of Physiology. 2009;587(1):195–210. doi: 10.1113/jphysiol.2008.159616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kidd M., Modlin I. M., Gustafsson B. I., Drozdov I., Hauso O., Pfragner R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. The American Journal of Physiology—Gastrointestinal and Liver Physiology. 2008;295(2):G260–G272. doi: 10.1152/ajpgi.00056.2008. [DOI] [PubMed] [Google Scholar]
- 24.Ren X., Zhou L., Terwilliger R., Newton S. S., de Araujo I. E. Sweet taste signaling functions as a hypothalamic glucose sensor. Frontiers in Integrative Neuroscience. 2009;3, article 12 doi: 10.3389/neuro.07.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gore F. M., Bloem P. J. N., Patton G. C., et al. Global burden of disease in young people aged 10–24 years: a systematic analysis. The Lancet. 2011;377(9783):2093–2102. doi: 10.1016/s0140-6736(11)60512-6. [DOI] [PubMed] [Google Scholar]
- 26.Johnston M. V., Alemi L., Harum K. H. Learning, memory, and transcription factors. Pediatric Research. 2003;53(3):369–374. doi: 10.1203/01.pdr.0000049517.47493.e9. [DOI] [PubMed] [Google Scholar]
- 27.Degenhardt L., Whiteford H. A., Ferrari A. J., et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. The Lancet. 2013;382(9904):1564–1574. doi: 10.1016/s0140-6736(13)61530-5. [DOI] [PubMed] [Google Scholar]
- 28.Schiöth H. B., Craft S., Brooks S. J., Frey W. H., II, Benedict C. Brain insulin signaling and Alzheimer's disease: current evidence and future directions. Molecular Neurobiology. 2012;46(1):4–10. doi: 10.1007/s12035-011-8229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de la Monte S. M. Insulin resistance and Alzheimer's disease. BMB Reports. 2009;42(8):475–481. doi: 10.5483/bmbrep.2009.42.8.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freiherr J., Hallschmid M., Frey W. H., II, et al. Intranasal insulin as a treatment for alzheimer's disease: a review of basic research and clinical evidence. CNS Drugs. 2013;27(7):505–514. doi: 10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wimo A., Prince M. World Alzheimer Report 2010: The Global Economic Impact of Dementia. Alzheimer’s Disease International; 2010. [Google Scholar]
- 32.Jönsson L., Wimo A. The cost of dementia in europe: a review of the evidence, and methodological considerations. PharmacoEconomics. 2009;27(5):391–403. doi: 10.2165/00019053-200927050-00004. [DOI] [PubMed] [Google Scholar]
- 33.Rockwood K., Brown M., Merry H., Sketris I., Fisk J. Societal costs of vascular cognitive impairment in older adults. Stroke. 2002;33(6):1605–1609. doi: 10.1161/01.str.0000017878.85274.44. [DOI] [PubMed] [Google Scholar]
- 34.Vossius C., Larsen J. P., Janvin C., Aarsland D. The economic impact of cognitive impairment in Parkinson's disease. Movement Disorders. 2011;26(8):1541–1544. doi: 10.1002/mds.23661. [DOI] [PubMed] [Google Scholar]
- 35.Alzheimer's Association. 2013 Alzheimer's disease facts and figures. Alzheimers & Dementia. 2013;9(2):208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Ernst R. L., Hay J. W. The US economic and social costs of Alzheimer's disease revisited. The American Journal of Public Health. 1994;84(8):1261–1264. doi: 10.2105/ajph.84.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newson B., Ahlman H., Dahlstrom A., Nyhus L. M. Ultrastructural observations in the rat ileal mucosa of possible epithelial ‘taste cells’ and submucosal sensory neurons. Acta Physiologica Scandinavica. 1982;114(2):161–164. doi: 10.1111/j.1748-1716.1982.tb06967.x. [DOI] [PubMed] [Google Scholar]
- 38.Margolskee R. F. Molecular mechanisms of bitter and sweet taste transduction. The Journal of Biological Chemistry. 2002;277(1):1–4. doi: 10.1074/jbc.r100054200. [DOI] [PubMed] [Google Scholar]
- 39.Lindemann B. Chemoreception: tasting the sweet and the bitter. Current Biology. 1996;6(10):1234–1237. doi: 10.1016/s0960-9822(96)00704-x. [DOI] [PubMed] [Google Scholar]
- 40.Margolskee R. F. The molecular biology of taste transduction. BioEssays. 1993;15(10):645–650. doi: 10.1002/bies.950151003. [DOI] [PubMed] [Google Scholar]
- 41.Shin Y.-K., Martin B., Golden E., et al. Modulation of taste sensitivity by GLP-1 signaling. Journal of Neurochemistry. 2008;106(1):455–463. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drucker D. J., Lovshin J., Baggio L., et al. New developments in the biology of the glucagon-like peptides GLP-1 and GLP-2. Annals of the New York Academy of Sciences. 2000;921:226–232. doi: 10.1111/j.1749-6632.2000.tb06970.x. [DOI] [PubMed] [Google Scholar]
- 43.Nakagawa Y., Nagasawa M., Yamada S., et al. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS ONE. 2009;4(4) doi: 10.1371/journal.pone.0005106.e5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohkuri T., Yasumatsu K., Horio N., Jyotaki M., Margolskee R. F., Ninomiya Y. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. The American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 2009;296(4):R960–R971. doi: 10.1152/ajpregu.91018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imada T., Misaka T., Fujiwara S., Okada S., Fukuda Y., Abe K. Amiloride reduces the sweet taste intensity by inhibiting the human sweet taste receptor. Biochemical and Biophysical Research Communications. 2010;397(2):220–225. doi: 10.1016/j.bbrc.2010.05.088. [DOI] [PubMed] [Google Scholar]
- 46.White H., Venkatesh B. Clinical review: ketones and brain injury. Critical Care. 2011;15(2, article 219) doi: 10.1186/cc10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida R., Niki M., Jyotaki M., Sanematsu K., Shigemura N., Ninomiya Y. Modulation of sweet responses of taste receptor cells. Seminars in Cell and Developmental Biology. 2013;24(3):226–231. doi: 10.1016/j.semcdb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Berg J. M., Tymoczko J. L., Stryer L. Biochemistry. 7th. New York, NY, USA: WH Freeman; 2010. [Google Scholar]
- 49.Welcome M. O., Razvodovsky Y. E., Pereverzeva E. V., Pereverzev V. A. State of Cognitive Functions of Students-Medics with Different Relationship to Alcohol Use. Minsk, Belarus: Belarusian State Medical University Press; 2013. [DOI] [PubMed] [Google Scholar]
- 50.Welcome M. O., Pereverzev V. A. Glucose memory facilitation effect. Swiss Journal of Medical Sciences. 2013;2(1):41–52. [Google Scholar]
- 51.Welcome M. O., Pereverzev V. A. Basal ganglia and the error monitoring and processing system: how alcohol modulates the error monitoring and processing capacity of the Basal ganglia. In: Barrios F. A., Bauer C., editors. Basal Ganglia—An Integrative View. InTech; 2013. pp. 65–86. [DOI] [Google Scholar]
- 52.Ding F., Yao J., Rettberg J. R., Chen S., Brinton R. D. Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and Alzheimer's mouse brain: implication for bioenergetic intervention. PLoS ONE. 2013;8(11) doi: 10.1371/journal.pone.0079977.e79977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prins M. L. Cerebral ketone metabolism during development and injury. Epilepsy Research. 2012;100(3):218–223. doi: 10.1016/j.eplepsyres.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welcome M. O., Pereverzeva E. V., Pereverzev V. A. A novel psychophysiological model of the effect of alcohol use on academic performance of male medical students of belarusian state medical university. International Journal of Collaborative Research on Internal Medicine and Public Health. 2010;2(6):183–197. [Google Scholar]
- 55.McNay E. C., Sherwin R. S. Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes. 2004;53(2):418–425. doi: 10.2337/diabetes.53.2.418. [DOI] [PubMed] [Google Scholar]
- 56.McNay E. C., Cotero V. E. Mini-review: impact of recurrent hypoglycemia on cognitive and brain function. Physiology and Behavior. 2010;100(3):234–238. doi: 10.1016/j.physbeh.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hill J., Zhao J., Dash P. K. High blood glucose does not adversely affect outcome in moderately brain-injured rodents. Journal of Neurotrauma. 2010;27(8):1439–1448. doi: 10.1089/neu.2010.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tallroth G., Ryding E., Agardh C.-D. Regional cerebral blood flow in normal man during insulin-induced hypoglycemia and in the recovery period following glucose infusion. Metabolism: Clinical and Experimental. 1992;41(7):717–721. doi: 10.1016/0026-0495(92)90310-7. [DOI] [PubMed] [Google Scholar]
- 59.Litvin M., Clark A. L., Fisher S. J. Recurrent hypoglycemia: boosting the brain's metabolic flexibility. Journal of Clinical Investigation. 2013;123(5):1922–1924. doi: 10.1172/jci69796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Frontiers in Human Neuroscience. 2009;3, article 31 doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welcome M. O., Pereverzev V. A. A mini-review of the mechanisms of glucose memory enhancement. International Journal of Medical and Pharmaceutical Sciences. 2013;4(1):17–30. [Google Scholar]
- 62.Levin B. E. Metabolic sensors: viewing glucosensing neurons from a broader perspective. Physiology and Behavior. 2002;76(3):397–401. doi: 10.1016/s0031-9384(02)00763-1. [DOI] [PubMed] [Google Scholar]
- 63.Levin B. E., Magnan C., Dunn-Meynell A., le Foll C. Metabolic sensing and the brain: who, what, where, and how? Endocrinology. 2011;152(7):2552–2557. doi: 10.1210/en.2011-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robertson D. A., Gernsbacher M. A., Guidotti S. J., et al. Functional neuroanatomy of the cognitive process of mapping during discourse comprehension. Psychological Science. 2000;11(3):255–260. doi: 10.1111/1467-9280.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahn H.-J., Seo S. W., Chin J., et al. The cortical neuroanatomy of neuropsychological deficits in mild cognitive impairment and Alzheimer's disease: a surface-based morphometric analysis. Neuropsychologia. 2011;49(14):3931–3945. doi: 10.1016/j.neuropsychologia.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 66.Svoboda E., McKinnon M. C., Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44(12):2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sykaras A. G., Demenis C., Case R. M., McLaughlin J. T., Smith C. P. Duodenal enteroendocrine I-cells contain mRNA transcripts encoding key endocannabinoid and fatty acid receptors. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0042373.e42373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bak L. K., Schousboe A., Waagepetersen H. S. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. Journal of Neurochemistry. 2006;98(3):641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- 69.Rowley N. M., Madsen K. K., Schousboe A., Steve White H. S. Glutamate and GABA synthesis, release, transport and metabolism as targets for seizure control. Neurochemistry International. 2012;61(4):546–558. doi: 10.1016/j.neuint.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Pascual J. M., Carceller F., Roda J. M., Cerdán S. Glutamate, glutamine, and GABA as substrates for the neuronal and glial compartments after focal cerebral ischemia in rats. Stroke. 1998;29(5):1048–1057. doi: 10.1161/01.str.29.5.1048. [DOI] [PubMed] [Google Scholar]
- 71.Mathews G. C., Diamond J. S. Neuronal glutamate uptake contributes to GABA synthesis and inhibitory synaptic strength. The Journal of Neuroscience. 2003;23(6):2040–2048. doi: 10.1523/JNEUROSCI.23-06-02040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schousboe A., Bak L. K., Waagepetersen H. S. Astrocytic control of biosynthesis and turnover of the neurotransmitters glutamate and GABA. Frontiers in Endocrinology. 2013;4, article 102 doi: 10.3389/fendo.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Andrews Z. B., Horvath T. L. Tasteless Food Reward. Neuron. 2008;57(6):806–808. doi: 10.1016/j.neuron.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 74.Takahashi H., Kato M., Takano H., et al. Differential contributions of prefrontal and hippocampal dopamine D1 and D2 receptors in human cognitive functions. The Journal of Neuroscience. 2008;28(46):12032–12038. doi: 10.1523/jneurosci.3446-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhuang Z., Yang B., Theus M. H., et al. EphrinBs regulate D-serine synthesis and release in astrocytes. The Journal of Neuroscience. 2010;30(47):16015–16024. doi: 10.1523/jneurosci.0481-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosenberg D., Kartvelishvily E., Shleper M., Klinker C. M. C., Bowser M. T., Wolosker H. Neuronal release of D-serine: a physiological pathway controlling extracellular D-serine concentration. The FASEB Journal. 2010;24(8):2951–2961. doi: 10.1096/fj.09-147967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Araujo I. E., Ren X., Ferreira J. G. Metabolic sensing in brain dopamine systems. Results and Problems in Cell Differentiation. 2010;52:69–86. doi: 10.1007/978-3-642-14426-4_7. [DOI] [PubMed] [Google Scholar]
- 78.Berga S. L., Loucks T. L. Use of cognitive behavior therapy for functional hypothalamic amenorrhea. Annals of the New York Academy of Sciences. 2006;1092:114–129. doi: 10.1196/annals.1365.010. [DOI] [PubMed] [Google Scholar]
- 79.Gan E. H., Pearce S. H. S. The thyroid in mind: cognitive function and low thyrotropin in older people. The Journal of Clinical Endocrinology and Metabolism. 2012;97(10):3438–3449. doi: 10.1210/jc.2012-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gasbarri A., Pompili A., Tavares M. C., Tomaz C. Estrogen and cognitive functions. Expert Review of Endocrinology & Metabolism. 2009;4(5):507–520. doi: 10.1586/eem.09.30. [DOI] [PubMed] [Google Scholar]
- 81.McEwen B. Estrogen actions throughout the brain. Recent Progress in Hormone Research. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 82.McEwen B. S., Alves S. E. Estrogen actions in the central nervous system. Endocrine Reviews. 1999;20(3):279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 83.Micevych P. E., Mermelstein P. G. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Molecular Neurobiology. 2008;38(1):66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leloup C., Arluison M., Lepetit N., et al. Glucose transporter 2 (GLUT 2): expression in specific brain nuclei. Brain Research. 1994;638(1-2):221–226. doi: 10.1016/0006-8993(94)90653-x. [DOI] [PubMed] [Google Scholar]
- 85.Sanz C., Roncero I., Alvarez E., Hurtado V., Blázquez E. Glucokinase as a glucose sensor in hypothalamus—regulation by orexigenic and anorexigenic peptides. In: Aimaretti G., editor. Update on Mechanisms of Hormone Action—Focus on Metabolism, Growth and Reproduction. Rijeka, Croatia: InTech; 2011. pp. 33–58. [Google Scholar]
- 86.Shetty P. K., Galeffi F., Turner D. A. Cellular links between neuronal activity and energy homeostasis. Frontiers in Pharmacology. 2012;3, article 43 doi: 10.3389/fphar.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lindsley J. E., Rutter J. Nutrient sensing and metabolic decisions. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2004;139(4):543–559. doi: 10.1016/j.cbpc.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 88.Rafalski V. A., Mancini E., Brunet A. Energy metabolism and energy-sensing pathways in mammalian embryonic and adult stem cell fate. Journal of Cell Science. 2012;125(23):5597–5608. doi: 10.1242/jcs.114827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deshmukh K., Anamika K., Srinivasan N. Evolution of domain combinations in protein kinases and its implications for functional diversity. Progress in Biophysics & Molecular Biology. 2010;102(1):1–15. doi: 10.1016/j.pbiomolbio.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 90.Bélanger M., Allaman I., Magistretti P. J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metabolism. 2011;14(6):724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 91.Hurtado-Carneiro V., Roncero I., Egger S. S., et al. PAS kinase is a nutrient and energy sensor in hypothalamic areas required for the normal function of AMPK and mTOR/S6K1. Molecular Neurobiology. 2014;50(2):314–326. doi: 10.1007/s12035-013-8630-4. [DOI] [PubMed] [Google Scholar]
- 92.Hurtado-Carneiro V., Sanz C., Roncero I., Vazquez P., Blazquez E., Alvarez E. Glucagon-like peptide 1 (GLP-1) can reverse AMP-activated protein kinase (AMPK) and S6 kinase (P70S6K) activities induced by fluctuations in glucose levels in hypothalamic areas involved in feeding behaviour. Molecular Neurobiology. 2012;45(2):348–361. doi: 10.1007/s12035-012-8239-z. [DOI] [PubMed] [Google Scholar]
- 93.Xu J., Ji J., Yan X.-H. Cross-talk between AMPK and mTOR in regulating energy balance. Critical Reviews in Food Science and Nutrition. 2012;52(5):373–381. doi: 10.1080/10408398.2010.500245. [DOI] [PubMed] [Google Scholar]
- 94.Ehninger D., de Vries P. J., Silva A. J. From mTOR to cognition: molecular and cellular mechanisms of cognitive impairments in tuberous sclerosis. Journal of Intellectual Disability Research. 2009;53(10):838–851. doi: 10.1111/j.1365-2788.2009.01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kyosseva S. V. The role of the extracellular signal-regulated kinase pathway in cerebellar abnormalities in schizophrenia. Cerebellum. 2004;3(2):94–99. doi: 10.1080/14734220410029164. [DOI] [PubMed] [Google Scholar]
- 96.David Sweatt J. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. Journal of Neurochemistry. 2001;76(1):1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 97.Xin X., Khan Z. A., Chen S., Chakrabarti S. Extracellular signal-regulated kinase (ERK) in glucose-induced and endothelin-mediated fibronectin synthesis. Laboratory Investigation. 2004;84(11):1451–1459. doi: 10.1038/labinvest.3700178. [DOI] [PubMed] [Google Scholar]
- 98.Gault V. A., Hölscher C. Protease-resistant glucose-dependent insulinotropic polypeptide agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta-amyloid. Journal of Neurophysiology. 2008;99(4):1590–1595. doi: 10.1152/jn.01161.2007. [DOI] [PubMed] [Google Scholar]
- 99.Tang S. J., Reis G., Kang H., Gingras A.-C., Sonenberg N., Schuman E. M. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adams J. P., Robinson R. A., Hudgins E. D., Wissink E. M., Dudek S. M. NMDA receptor-independent control of transcription factors and gene expression. NeuroReport. 2009;20(16):1429–1433. doi: 10.1097/wnr.0b013e3283311db6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zorumski C. F., Izumi Y. Modulation of LTP induction by NMDA receptor activation and nitric oxide release. Progress in Brain Research. 1998;118:173–182. doi: 10.1016/s0079-6123(08)63207-0. [DOI] [PubMed] [Google Scholar]
- 102.Godfraind J.-M., Xu Y.-Z. Two-deoxyglucose-induced long-term potentiation in slices of rat dentrate gyrus. Critical Reviews in Neurobiology. 2006;18(1-2):37–48. doi: 10.1615/critrevneurobiol.v18.i1-2.50. [DOI] [PubMed] [Google Scholar]
- 103.Puighermanal E., Busquets-Garcia A., Maldonado R., Ozaita A. Cellular and intracellular mechanisms involved in the cognitive impairment of cannabinoids. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367(1607):3254–3263. doi: 10.1098/rstb.2011.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang H., Zhuo M. Group I metabotropic glutamate receptor-mediated gene transcription and implications for synaptic plasticity and diseases. Frontiers in Pharmacology. 2012;3, article 189 doi: 10.3389/fphar.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tardito D., Perez J., Tiraboschi E., Musazzi L., Racagni G., Popoli M. Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: a critical overview. Pharmacological Reviews. 2006;58(1):115–134. doi: 10.1124/pr.58.1.7. [DOI] [PubMed] [Google Scholar]
- 106.Wiegert J. S., Bading H. Activity-dependent calcium signaling and ERK-MAP kinases in neurons: a link to structural plasticity of the nucleus and gene transcription regulation. Cell Calcium. 2011;49(5):296–305. doi: 10.1016/j.ceca.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 107.Alberini C. M. Transcription factors in long-term memory and synaptic plasticity. Physiological Reviews. 2009;89(1):121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beckmann A. M., Wilce P. A. Egr transcription factors in the nervous system. Neurochemistry International. 1997;31(4):477–510. doi: 10.1016/s0197-0186(96)00136-2. [DOI] [PubMed] [Google Scholar]
- 109.Bernal-Mizrachi E., Wen W., Srinivasan S., Klenk A., Cohen D., Alan Permutt M. Activation of Elk-1, an Ets transcription factor, by glucose and EGF treatment of insulinoma cells. American Journal of Physiology: Endocrinology and Metabolism. 2001;281(6):E1286–E1299. doi: 10.1152/ajpendo.2001.281.6.E1286. [DOI] [PubMed] [Google Scholar]
- 110.Herdegen T., Skene P., Bähr M. The c-Jun transcription factor—bipotential mediator of neuronal death, survival and regeneration. Trends in Neurosciences. 1997;20(5):227–231. doi: 10.1016/s0166-2236(96)01000-4. [DOI] [PubMed] [Google Scholar]
- 111.Xu X., Zheng X. Potential involvement of calcium and nitric oxide in protective effects of puerarin on oxygen-glucose deprivation in cultured hippocampal neurons. Journal of Ethnopharmacology. 2007;113(3):421–426. doi: 10.1016/j.jep.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 112.Canabal D. D., Potian J. G., Duran R. G., McArdle J. J., Routh V. H. Hyperglycemia impairs glucose and insulin regulation of nitric oxide production in glucose-inhibited neurons in the ventromedial hypothalamus. American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 2007;293(2):R592–R600. doi: 10.1152/ajpregu.00207.2007. [DOI] [PubMed] [Google Scholar]
- 113.Kandel E. R. The molecular biology of memory storage: a dialog between genes and synapses. Bioscience Reports. 2001;21(5):565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- 114.Okuno H. Regulation and function of immediate-early genes in the brain: beyond neuronal activity markers. Neuroscience Research. 2011;69(3):175–186. doi: 10.1016/j.neures.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 115.Sweatt J. D. Mechanisms of Memory. Boston, Mass, USA: Academic Press; 2003. [Google Scholar]
- 116.Fowler T., Sen R., Roy A. L. Regulation of primary response genes. Molecular Cell. 2011;44(3):348–360. doi: 10.1016/j.molcel.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Loebrich S., Nedivi E. The function of activity-regulated genes in the nervous system. Physiological Reviews. 2009;89(4):1079–1103. doi: 10.1152/physrev.00013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Craddock T. J. A., Tuszynski J. A., Hameroff S. Cytoskeletal signaling: is memory encoded in microtubule lattices by CaMKII phosphorylation? PLoS Computational Biology. 2012;8(3) doi: 10.1371/journal.pcbi.1002421.e1002421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cassimeris L. Microtubule associated proteins in neurons. Encyclopedia of Neuroscience. 2010:865–870. doi: 10.1016/b978-008045046-9.00725-7. [DOI] [Google Scholar]
- 120.Cash A. D., Aliev G., Siedlak S. L., et al. Microtubule reduction in Alzheimer's disease and aging is independent of τ filament formation. The American Journal of Pathology. 2003;162(5):1623–1627. doi: 10.1016/s0002-9440(10)64296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu X., Zhou J., Abid M. D. N., et al. Berberine attenuates axonal transport impairment and axonopathy induced by calyculin a in N2a cells. PLoS ONE. 2014;9(4) doi: 10.1371/journal.pone.0093974.e93974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bibb J. A., Mayford M. R., Tsien J. Z., Alberini C. M. Cognition enhancement strategies. The Journal of Neuroscience. 2010;30(45):14987–14992. doi: 10.1523/jneurosci.4419-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moriguchi S., Han F., Nakagawasai O., Tadano T., Fukunaga K. Decreased calcium/calmodulin-dependent protein kinase II and protein kinase C activities mediate impairment of hippocampal long-term potentiation in the olfactory bulbectomized mice. Journal of Neurochemistry. 2006;97(1):22–29. doi: 10.1111/j.1471-4159.2006.03710.x. [DOI] [PubMed] [Google Scholar]
- 124.Dent E. W., Baas P. W. Microtubules in neurons as information carriers. Journal of Neurochemistry. 2014;129(2):235–239. doi: 10.1111/jnc.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baas P. W., Yu W. A composite model for establishing the microtubule arrays of the neuron. Molecular Neurobiology. 1996;12(2):145–161. doi: 10.1007/BF02740651. [DOI] [PubMed] [Google Scholar]
- 126.Georgiev D. Remarks on the number of tubulin dimers per neuron and implications for Hameroff-Penrose Orch OR. NeuroQuantology. 2009;7(4):677–679. [Google Scholar]
- 127.Craddock T. J. A., Tuszynski J. A. A critical assessment of the information processing capabilities of neuronal microtubules using coherent excitations. Journal of Biological Physics. 2010;36(1):53–70. doi: 10.1007/s10867-009-9158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Neubert F.-X., Mars R. B., Thomas A. G., Sallet J., Rushworth M. F. S. Comparison of human ventral frontal cortex areas for cognitive control and language with areas in monkey frontal cortex. Neuron. 2014;81(3):700–713. doi: 10.1016/j.neuron.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 129.Snowball A., Tachtsidis I., Popescu T., et al. Long-term enhancement of brain function and cognition using cognitive training and brain stimulation. Current Biology. 2013;23(11):987–992. doi: 10.1016/j.cub.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cabeza R., Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 131.Peyron R., Laurent B., García-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiologie Clinique. 2000;30(5):263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 132.Sergent J. Brain-imaging studies of cognitive functions. Trends in Neurosciences. 1994;17(6):221–227. doi: 10.1016/0166-2236(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 133.Fridriksson J., Guo D., Fillmore P., Holland A., Rorden C. Damage to the anterior arcuate fasciculus predicts non-fluent speech production in aphasia. Brain. 2013;136(11):3451–3460. doi: 10.1093/brain/awt267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Motta S. C., Goto M., Gouveia F. V., Baldo M. V. C., Canteras N. S., Swanson L. W. Dissecting the brain's fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(12):4870–4875. doi: 10.1073/pnas.0900939106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Giles D. E., Berga S. L. Cognitive and psychiatric correlates of functional hypothalamic amenorrhea: a controlled comparison. Fertility and Sterility. 1993;60(3):486–492. [PubMed] [Google Scholar]
- 136.Gahete M. D., Córdoba-Chacón J., Kineman R. D., Luque R. M., Castaño J. P. Role of ghrelin system in neuroprotection and cognitive functions: implications in Alzheimer's disease. Peptides. 2011;32(11):2225–2228. doi: 10.1016/j.peptides.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Prigatano G. P. Cognitive and behavioral dysfunction in children with hypothalamic hamartoma and epilepsy. Seminars in Pediatric Neurology. 2007;14(2):65–72. doi: 10.1016/j.spen.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 138.Morrison C. D. Leptin signaling in brain: a link between nutrition and cognition? Biochimica et Biophysica Acta: Molecular Basis of Disease. 2009;1792(5):401–408. doi: 10.1016/j.bbadis.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nyberg F., Hallberg M. Growth hormone and cognitive function. Nature Reviews Endocrinology. 2013;9(6):357–365. doi: 10.1038/nrendo.2013.78. [DOI] [PubMed] [Google Scholar]
- 140.Shin Y.-K., Egan J. M. Roles of hormones in taste signaling. Results and Problems in Cell Differentiation. 2010;52:115–137. doi: 10.1007/978-3-642-14426-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sparks D. L., DeKosky S. T., Markesbery W. R. Alzheimer's disease: aminergic-cholinergic alterations in hypothalamus. Archives of Neurology. 1988;45(9):994–999. doi: 10.1001/archneur.1988.00520330084014. [DOI] [PubMed] [Google Scholar]
- 142.Shah K., DeSilva S., Abbruscato T. The role of glucose transporters in brain disease: diabetes and Alzheimer's disease. International Journal of Molecular Sciences. 2012;13(10):12629–12655. doi: 10.3390/ijms131012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Temussi P. The sweet taste receptor: a single receptor with multiple sites and modes of interaction. Advances in Food and Nutrition Research. 2007;53:199–239. doi: 10.1016/s1043-4526(07)53006-8. [DOI] [PubMed] [Google Scholar]
- 144.Cui M., Jiang P., Maillet E., Max M., Margolskee R. E., Osman R. The heterodimeric sweet taste receptor has multiple potential ligand binding sites. Current Pharmaceutical Design. 2006;12(35):4591–4600. doi: 10.2174/138161206779010350. [DOI] [PubMed] [Google Scholar]
- 145.Morini G., Bassoli A., Temussi P. A. From small sweeteners to sweet proteins: anatomy of the binding sites of the human T1R2_T1R3 receptor. Journal of Medicinal Chemistry. 2005;48(17):5520–5529. doi: 10.1021/jm0503345. [DOI] [PubMed] [Google Scholar]
- 146.Kinnamon S. C. Taste receptor signalling—from tongues to lungs. Acta Physiologica. 2012;204(2):158–168. doi: 10.1111/j.1748-1716.2011.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pérez C. A., Huang L., Rong M., et al. A transient receptor potential channel expressed in taste receptor cells. Nature Neuroscience. 2002;5(11):1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 148.Taruno A., Vingtdeux V., Ohmoto M., et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013;495(7440):223–226. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huangu L., Shanker Y. G., Dubauskaite J., et al. Gγ13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nature Neuroscience. 1999;2(12):1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- 150.Yosuke M., Yuko N., Jinhui M., et al. A model for signal transduction mechanism downstream of the sweet taste-sensing receptors in taste cells and 3T3-L1 cells. PLoS ONE. 2013 doi: 10.1371/journal.pone.0054500.g006. [DOI] [Google Scholar]
- 151.Clapp T. R., Trubey K. R., Vandenbeuch A., et al. Tonic activity of Gα-gustducin regulates taste cell responsivity. FEBS Letters. 2008;582(27):3783–3787. doi: 10.1016/j.febslet.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Murata Y., Yasuo T., Yoshida R., et al. Action potential-enhanced ATP release from taste cells through hemichannels. Journal of Neurophysiology. 2010;104(2):896–901. doi: 10.1152/jn.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McLaughlin S. K., McKinnon P. J., Robichon A., Spickofsky N., Margolskee R. F. Gustducin and transducin: a tale of two G proteins. Ciba Foundation Symposium. 1993;179:186–196. doi: 10.1002/9780470514511.ch12. [DOI] [PubMed] [Google Scholar]
- 154.Welcome M. O., Pereverzev V. A. Glycemia and memory. In: Szablewski L., editor. Glucose Homeostasis. Rijeka, Croatia: InTech; 2014. pp. 113–130. [Google Scholar]