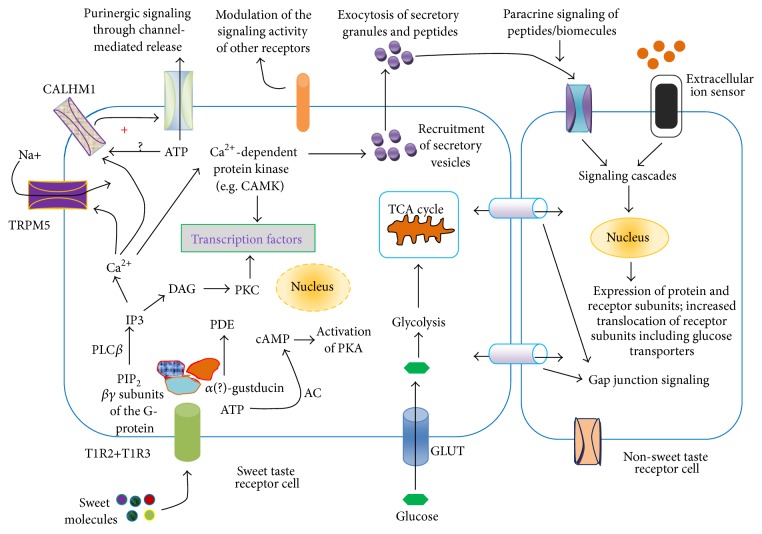
Figure 1.
A general model of sweet taste signaling network. Sweet taste receptors possess multiple binding sites and mode of interaction for sweet molecules and they belong to class C of heterotrimeric guanine nucleotide-binding protein, G-protein [143–145]. Sweet molecules activate the G-protein by downstream signaling leading to the dissociation of the α-subunit gustducin from the βγ subunits [146, 147]. Dissociated βγ subunits of the G-protein activate phospholipase Cβ (PLCβ), leading to the formation of 1,4,5-inositol trisphosphate (IP3). IP3 is responsible for the release of Ca2+ from intracellular stores through its binding to IP3-receptor in these stores. Increase in intracellular Ca2+ activates calcium dependent kinase, monovalent selective cation channel, TRPM5 (transient receptor potential cation channel, subfamily M, member 5) [15, 44, 146], and other receptors [44, 148]. To establish the role of TRPM5 or PLCβ (type 2), Zhang et al. [4] showed that knockout of the receptor or the enzyme abolishes the sensation of taste in cells. TRPM5 may also play a role in capacitance mediated calcium entry into taste cells [147]. Modulation of purinergic signaling by taste receptor also plays useful role in taste sensation. For the initiation of purinergic release, it was recently demonstrated by Taruno et al. [148] that the voltage-gated ion channel, calcium homeostasis modulator 1 (CALHM1), is indispensable for taste-stimuli-evoked ATP release from sweet, bitter, and umami taste cells. Importantly, CALHM1 is expressed not only in sweet but also in bitter and umami taste sensing type 2 cells. Taruno et al. [148] proposed that CALHM1 is a voltage-gated ATP-release channel. Dissociated α subunit referred to as Gα-gustducin activates a phosphodiesterase (PDE) thereby decreasing intracellular cAMP levels [146, 149]. Gα-gustducin is also reported to activate adenylate cyclase (AC) to increase cAMP level [150]. According to earlier report, Clapp et al. [151] demonstrated that, compared to wild type mice, knockout of α-gustducin in the taste buds of mice resulted in high basal (unstimulated) cAMP level. The results of these authors [151] indicated that α-gustducin is necessary to maintain low level of cAMP level. Low level of cAMP is necessary to maintain the adequate signaling of Ca2+ by disinhibition of cyclic nucleotide-inhibited channels to elevate intracellular Ca2+ [38]. Changes in cAMP levels also affect other ion channels, including K+ channels. The events resulting in activation/modulation of ion channels lead to membrane depolarization and formation of action potentials. Potential-dependent release of mediators (ATP, serotonin, etc.) and peptides and calcium dependent release of peptides/biomolecules are some of the results of sweet taste receptor signaling [152]. A hallmark of sweet taste receptor signaling is the activation of transcription factors and gene expression, which might be dependent on calcium and activity dependent activation calcium dependent kinases, including the calmodulin-dependent protein kinase (CAMK). Activation of protein kinases may be achieved through other signaling pathways. It appears that sweet taste receptor signaling involves multiple activating substrates and different types and subtypes of both α-gustducin and βγ subunits of the G-protein. Although, different subtypes of sweet taste G-protein receptor subunits have been known for over a decade, their specific roles in sensing taste are not exactly clear [38, 149, 153]. For instance, Huangu et al. [149] reported the presence of β1 and γ13. The sweet taste receptor is also known to have β3 subtype subunit. For α-gustducin, Gα i-2, Gα i-3, Gα 14, Gα 15, Gα q, Gα s, α-transducin have been identified [38].