Figure 2.
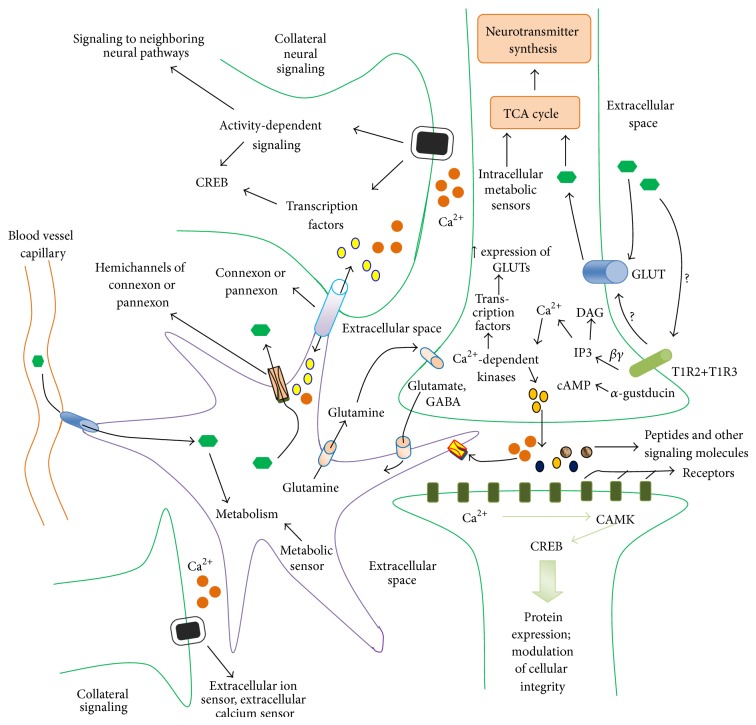
Sweet taste signaling network of the neuroastroglial system. The brain is a complex network of cells, largely populated by neurons and astrocytes. There are ~100 billion neurons, with glial cells outnumbering neurons by about 10-fold. Astrocytes form the largest population of glial cells. The metabolic role of astrocytes in brain has been reviewed in our previous work [60]. Mechanisms of how glucose enters into the astrocytes and neurons are well documented [61]. From the scheme (Figure 2), the presynaptic neuron senses glucose mediated by the T1R2+T1R3 and GLUT2. While the mechanisms, by which the neuron senses glucose through GLUT2, have increasingly been defined, the glucosensing mechanisms of the sweet taste receptor are yet to be understood. It is quite possible that sweet taste receptor can modulate the plasma membrane GLUT2 glucosensor. Functioning cooperatively with GLUT2 to sense the metabolic rate of the intracellular milieu is the G-protein coupled receptor, the inwardly rectifying ATP-dependent potassium channel (KATP channel) [62, 63]. Transport activity of GLUT2 may be affected through multiple signaling pathways, such as those involving the regulation of GLUT2 and KATP channel activity. While in Ren et al. [24] study, the signaling activity of GLUT2 was not assessed, their results showed that the inhibition of sweet taste receptor resulted in increase in taste receptor gene expression, suggesting that sweet taste receptors persistently code information about the extracellular glucose level to intracellular milieu, and this might, probably, involve intracellular metabolic sensors, mediating neural activity, gene expression, and membrane receptor trafficking. Although, the mechanisms of the T1R2+T1R3/GLUT2-cooperativity/associativity (or intracellular metabolic sensors) interaction are not known, it can be proposed that T1R2+T1R3 could modulate GLUT2 transport activity through mechanisms as yet unknown. Mechanism of downstream signaling of the neuronal T1R2+T1R3 receptor is similar to that in other cells (Figure 1). The downstream signaling of these receptors can result in changes in extracellular calcium concentration as well as changes in peptide secretions. These biomolecules are sensed by their corresponding receptors in the adjacent neurons/astrocytes, which couple the received information into intracellular signal and cellular activity. The waves of calcium ions, peptide-dependent signaling, can affect collateral neurons and astrocytes by activity dependent signaling and changes in ion waves and regulate gene expression and protein synthesis. Several transcription factors and memory relation genes are activated/deactivated. Intercellular signaling through connexons and pannexons in these cells can modulate their activity.