Abstract
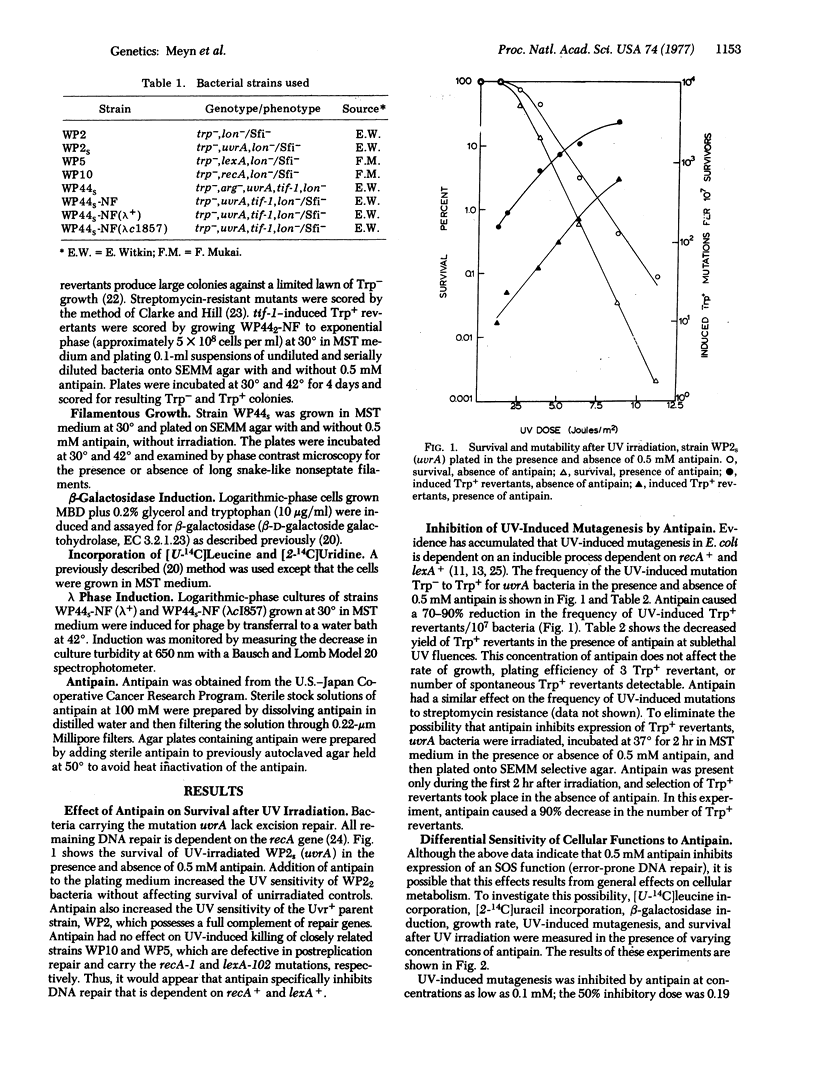
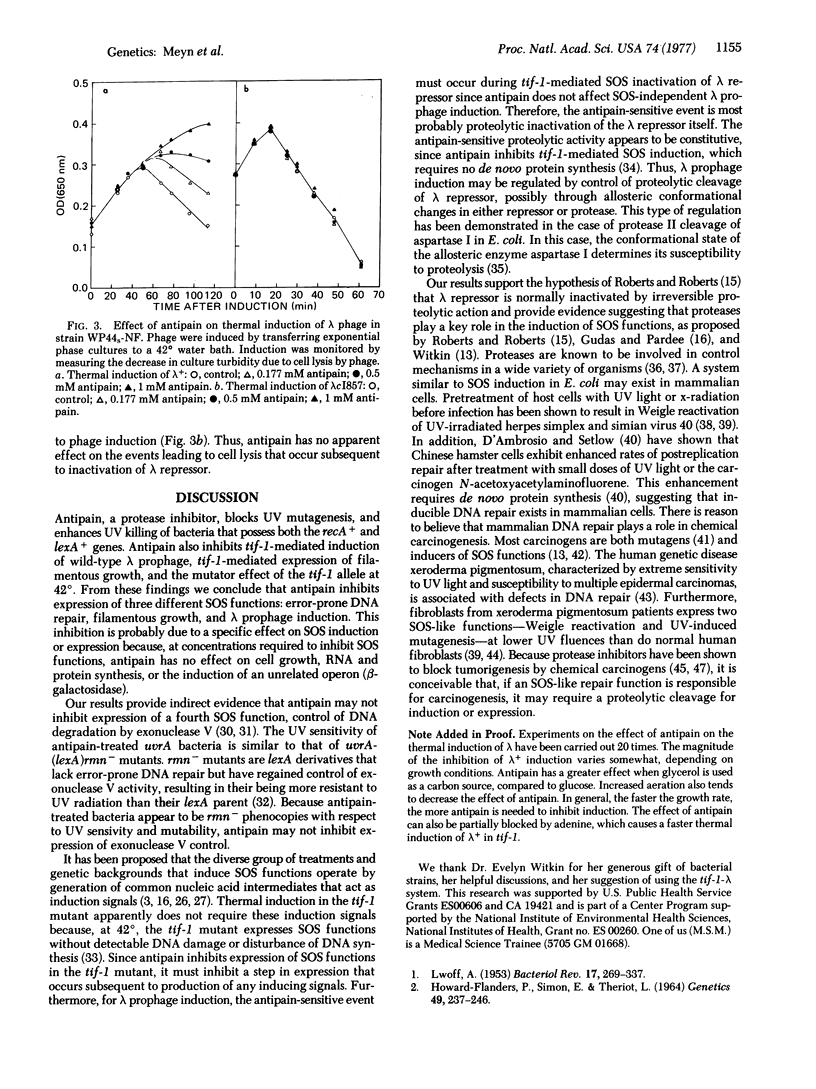
Inhibition of DNA synthesis in E. coli by treatment with carcinogenic and mutagenic agents results in the coordinate expression of a group of diverse functions (SOS functions) including lambda prophage induction, filamentous growth, and an error-prone DNA repair activity (SOS repair) believed to be responsible for ultraviolet mutagenesis. It has been proposed that this SOS induction proceeds via irreversible proteolytic inactivation of repressor(s) for SOS functions. To test this hypothesis, we investigated the effect of a protease inhibitor, antipain [(1-carboxy-2-phenylethyl)carbamoyl-L-arginyl-L-valylargininal], on SOS induction. We found that 0.5 mM antipain (which has no effect on cell growth, overall RNA and protein synthesis, or induction of beta-galactosidase) drastically decreases mutagenesis. Antipain also blocks expression of thermally induced mutator activity (another manifestation of SOS repair) and filamentous growth in a tif-1 mutant that expresses SOS functions at 42 degrees without inhibition of DNA synthesis or detectable DNA damage. Furthermore, antipain inhibits thermal induction of lambda prophage in the tif-1 mutant without affecting the kinetics of thermal induction of lambdacI857 prophage. This lambda mutant codes a temperature-sensitive repressor that is directly destroyed by heat and does not require the SOS induction pathway for inactivation at 42 degrees. From our results we conclude that antipain inhibits lambda prophage induction by blocking proteolytic inactivation of lambda repressor and that it inhibits the induction or expression of SOS repair and filamentous growth. Our results suggest a role for proteolytic cleavage in the regulation of SOS functions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Durston W. E., Yamasaki E., Lee F. D. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2281–2285. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockstahler L. E., Lytle C. D. Ultraviolet light enhanced reactivation of a mammalian virus. Biochem Biophys Res Commun. 1970 Oct 9;41(1):184–189. doi: 10.1016/0006-291x(70)90486-9. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Clarke C. H., Hill R. F. Mutation frequency decline for streptomycin-resistant mutations induced by ultraviolet light in Escherichia coli B-r. Mutat Res. 1972 Feb;14(2):247–249. doi: 10.1016/0027-5107(72)90051-6. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Bootsma D. Xeroderma pigmentosum: biochemical and genetic characteristics. Annu Rev Genet. 1975;9:19–38. doi: 10.1146/annurev.ge.09.120175.000315. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio S. M., Setlow R. B. Enhancement of postreplication repair in Chinese hamster cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2396–2400. doi: 10.1073/pnas.73.7.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defais M., Fauquet P., Radman M., Errera M. Ultraviolet reactivation and ultraviolet mutagenesis of lambda in different genetic systems. Virology. 1971 Feb;43(2):495–503. doi: 10.1016/0042-6822(71)90321-7. [DOI] [PubMed] [Google Scholar]
- GOLDTHWAIT D., JACOB F. SUR LE M'ECANISME DE L'INDUCTION DU D'EVELOPPEMENT DU PROPHAGE CHEZ LES BACT'ERIES LYSOG'ENES. C R Hebd Seances Acad Sci. 1964 Jul 20;259:661–664. [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- George J., Devoret R., Radman M. Indirect ultraviolet-reactivation of phage lambda. Proc Natl Acad Sci U S A. 1974 Jan;71(1):144–147. doi: 10.1073/pnas.71.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. Isolation of the lac repressor. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1891–1898. doi: 10.1073/pnas.56.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Model for regulation of Escherichia coli DNA repair functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2330–2334. doi: 10.1073/pnas.72.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Hozumi M., Ogawa M., Sugimura T., Takeuchi T., Umezawa H. Inhibition of tumorigenesis in mouse skin by leupeptin, a protease inhibitor from Actinomycetes. Cancer Res. 1972 Aug;32(8):1725–1728. [PubMed] [Google Scholar]
- Inouye M., Pardee A. B. Changes of membrane proteins and their relation to deoxyribonucleic acid synthesis and cell division of Escherichia coli. J Biol Chem. 1970 Nov 10;245(21):5813–5819. [PubMed] [Google Scholar]
- Kirby E. P., Jacob F., Goldthwait D. A. Prophage induction and filament formation in a mutant strain of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1903–1910. doi: 10.1073/pnas.58.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LWOFF A. Lysogeny. Bacteriol Rev. 1953 Dec;17(4):269–337. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle C. D., Day R. S., 3rd, Hellman K. B., Bockstahler L. E. Infection of UV-irradiated xeroderma pigmentosum fibroblasts by herpes simplex virus: study of capacity and Weigle reactivation. Mutat Res. 1976 Sep;36(3):257–264. doi: 10.1016/0027-5107(76)90235-9. [DOI] [PubMed] [Google Scholar]
- MELECHEN N. E., SKAAR P. D. The provocation of an early step of induction by thymine deprivation. Virology. 1962 Jan;16:21–29. doi: 10.1016/0042-6822(62)90198-8. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Pollard E. C., Ginoza W., Randall E. P. Involvement of recA and exr genes in the in vivo inhibition of the recBC nuclease. J Bacteriol. 1974 May;118(2):465–470. doi: 10.1128/jb.118.2.465-470.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P., Bailone A., Devoret R. Prophage lambda induction of Escherichia coli K12 envA uvrB: a highly sensitive test for potential carcinogens. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3700–3704. doi: 10.1073/pnas.73.10.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack D., Klaus S. Inactivation kinetics of lambda phage repressors in a mutant of E. coli temperature sensitive in DNA replication. Mol Gen Genet. 1972;115(3):216–224. doi: 10.1007/BF00268885. [DOI] [PubMed] [Google Scholar]
- Pacaud M., Richaud C. Protease II from Escherichia coli. Purification and characterization. J Biol Chem. 1975 Oct 10;250(19):7771–7779. [PubMed] [Google Scholar]
- Ptashne M. ISOLATION OF THE lambda PHAGE REPRESSOR. Proc Natl Acad Sci U S A. 1967 Feb;57(2):306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman T., Meyn M. S., Troll W. Effects of sodium arsenite on the survival of UV-irradiated Escherichia coli: inhibition of a recA-dependent function. Mutat Res. 1975 Nov;30(2):157–162. [PubMed] [Google Scholar]
- Rossman T., Norris C., Troll W. Inhibition of macromolecular synthesis in Escherichia coli by protease inhibitors. Specific reversal by glutathione of the effects of chloromethyl ketones. J Biol Chem. 1974 Jun 10;249(11):3412–3417. [PubMed] [Google Scholar]
- SUSSMAN R., JACOB F. [On a thermosensitive repression system in the Escherichia coli lambda bacteriophage]. C R Hebd Seances Acad Sci. 1962 Feb 19;254:1517–1519. [PubMed] [Google Scholar]
- Sedgwick S. G. Inducible error-prone repair in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2753–2757. doi: 10.1073/pnas.72.7.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolun A., Christensen R., Pollard E. C. Repair of radiation-induced strand breaks as related to the inducible inhibitor of postirradiation DNA degradation. Biophys J. 1974 Sep;14(9):691–696. doi: 10.1016/S0006-3495(74)85944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa S., Tatsuta K., Fujimoto K., Tsuchiya T., Umezawa H. Structure of antipain, a new Sakaguchi-positive product of streptomyces. J Antibiot (Tokyo) 1972 Apr;25(4):267–270. doi: 10.7164/antibiotics.25.267. [DOI] [PubMed] [Google Scholar]
- Volkert M. R., George D. L., Witkin E. M. Partial suppression of the LexA phenotype by mutations (rnm) which restore ultraviolet resistance but not ultraviolet mutability to Escherichia coli B/r uvr A lexA. Mutat Res. 1976 Jul;36(1):17–28. doi: 10.1016/0027-5107(76)90017-8. [DOI] [PubMed] [Google Scholar]
- Weigle J. J. Induction of Mutations in a Bacterial Virus. Proc Natl Acad Sci U S A. 1953 Jul;39(7):628–636. doi: 10.1073/pnas.39.7.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. C., Powell K. A., Emmerson P. T. recA+-dependent inactivation of the lambda repressor in Escherichia coli lysogens by gamma-radiation and by tif expression. Mol Gen Genet. 1975 Nov 3;141(1):1–8. doi: 10.1007/BF00332374. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. The radiation sensitivity of Escherichia coli B: a hypothesis relating filament formation and prophage induction. Proc Natl Acad Sci U S A. 1967 May;57(5):1275–1279. doi: 10.1073/pnas.57.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


