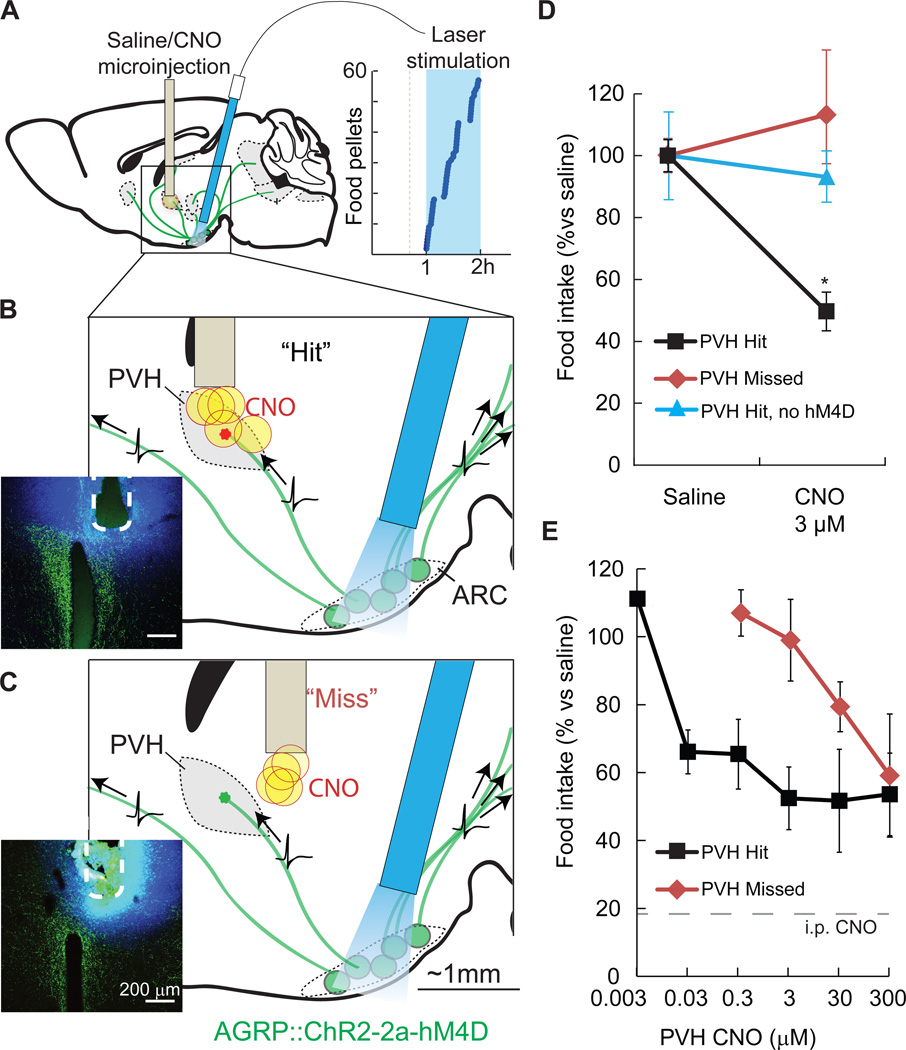
Figure 3. Spatially targeted synaptic silencing in vivo.
A–C) Schematics for photostimulation of AGRP neurons co-expressing ChR2 and hM4D in the arcuate nucleus (ARC), while spatially targeting CNO microinjection to the PVH. An angled optical fiber is implanted over the ARC to photostimulate AGRP neuron somata (green), which project axons to multiple brain areas. AGRP neuron photostimulation in this configuration robustly evoked feeding (right). CNO or saline were targeted to the PVH through an implanted cannula. (B) Focal microinjection of CNO to the PVH during AGRP neuron photoactivation result in selective silencing of synaptic release from the targeted ARCAGRP→PVH axonal projection field due to hM4D–mediated synaptic inhibition. Evoked food intake is only partially reduced because AGRP neuron axons still transmit action potentials, and non-targeted axon projections remain competent for synaptic transmission. Injection sites were verified postmortem (inset) by injection of FluoroGold (blue) and AGRP immunofluorescence (green). (C) In some mice, cannula placement was outside of the PVH by 300–500 µm (“Miss”).
D) Local microinjections of CNO into the PVH (“Hits”) significantly reduced feeding (3 µM CNO, n = 5). No reduction in feeding was observed for CNO injections that missed the PVH (3 µM CNO, n = 4) or with CNO injections into ChR2-expresing mice that lack hM4D (300 µM CNO, n = 5).
E) PVH microinjection of CNO reduced feeding by about 50% over a range of doses. Precision of targeted synaptic silencing was reduced with increasing CNO dose, as evidenced by the capacity of injections that miss the PVH to inhibit feeding. Intraperitoneal injection of CNO further reduced evoked feeding to basal levels (18 ± 4% of evoked feeding). Values are means ± SEM. *p < 0.05.