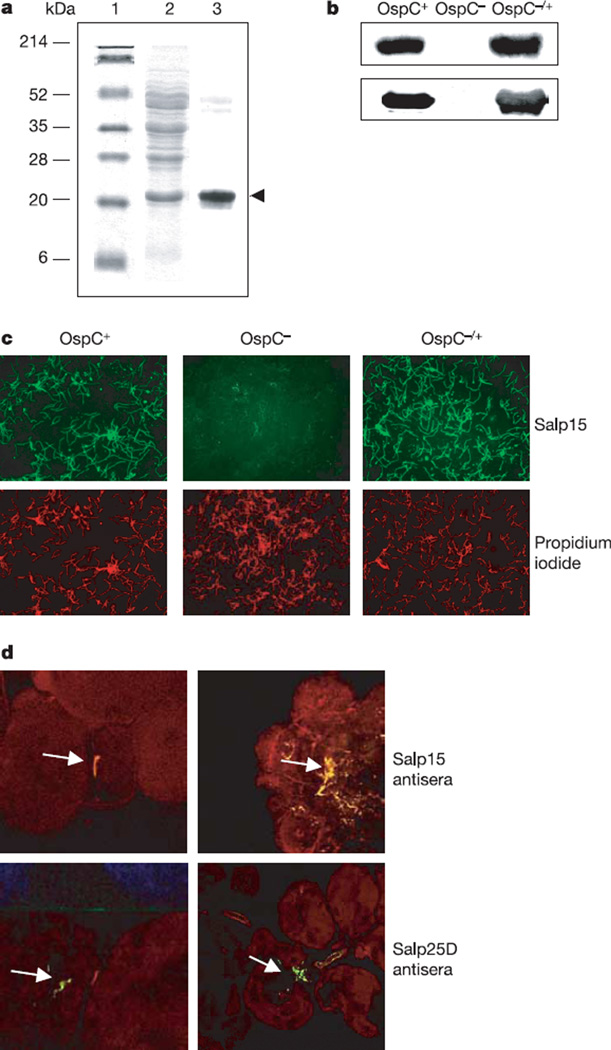
Figure 2. Salp15 interacts with outer surface protein (Osp)C of Borrelia burgdorferi.
a, Salp15 bound specifically to OspC. Lanes 1 and 2, Ponceau S stain of molecular mass markers (lane 1) and B. burgdorferi lysate (lane 2); lane 3, Salp15 overlay. The protein band bound by Salp15 (marked by an arrowhead) was identified as OspC. b, Salp15 binding to OspC was confirmed by using wild-type (OspC+), OspC-deficient (OspC−) and OspC-complemented (OspC−/+) B. burgdorferi. Bacterial lysates of the isolates were probed with anti-Salp15 (top) and anti-OspC (bottom) antibody. c, Salp15 binds to the surface of intact B. burgdorferi. Unfixed wild-type (OspC+), OspC-deficient (OspC−) and OspC-complemented (OspC−/+) B. burgdorferi were probed with FITC-conjugated Salp15 (green) and propidium iodide (red). Original magnification × 40. d, Salp15 binds B. burgdorferi within the tick salivary gland. Top: salivary glands from B. burgdorferi-infected nymphs were probed with Salp15 antibody (red). The spirochaetes (as indicated by arrows) were stained with an FITC-labelled anti-B. burgdorferi antibody (green). Bottom: anti-Salp25D served as a control. Co-localization (yellow) was observed with Salp15 antisera, in contrast to Salp25D. Left, salivary gland with a single spirochaete; right, a cluster of B. burgdorferi is harboured. Images are representative of ten independent experiments. Original magnification × 40.