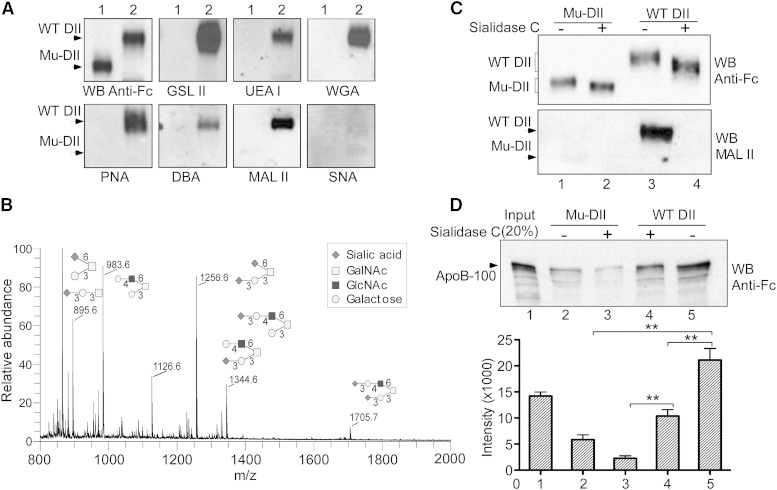
Fig. 5.
The structure and function analyses of perlecan DII O-linked glycans in LDL binding. A: Western blot analysis of WT and Mu-DII proteins with lectin probes. Equal amounts of the Fc-tagged Mu-DII and WT DII proteins, as in Fig. 4A, were analyzed on a gradient SDS-PAGE and probed with the anti-Fc antibody or the lectins, including GSL II (GlcNAc), UEA I (fucose), PNA (galactose), WGA (GlcNAc and sialic acid), DBA (GalNAc), MAL II, and SNA [(α2-3)- and (α2-6)-linked sialic acid, respectively]. B: Glycosylation profile of perlecan DII. O-linked glycans were released from purified Fc-tagged WT DII. The electrospray ionization-ion trap (ESI-IT) spectrum of O-linked glycans with possible isomers is shown. The symbols for each sugar are shown. C: Desialylation analysis of perlecan DII. The Fc-tagged WT DII and Mu-DII were treated with or without sialidase C and analyzed with Western blotting. Blots were probed with the Fc-specific antibody (top) or MAL II lectin (bottom). D: In vitro binding assay of desialylated DII in LDL binding. Top, Fc-tagged WT DII and Mu-DII treated with or without sialidase C, as in (C), were used for in vitro binding assay with human LDL (1.5 μg) as in Fig. 1E, and then analyzed with Western blotting. Blot was probed with the anti-ApoB antibody. Bottom, quantitation of the in vitro binding assay. The average and standard error were calculated from three independent experiments. **P < 0.01 (t-test).