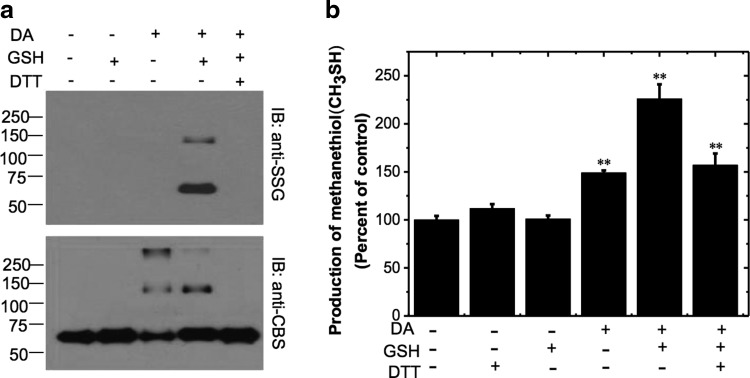
FIG. 1.
S-glutathionylation of CBS in vitro and its effect on activity. (a) Purified recombinant CBS (0.5 mg/ml) was incubated with 1 mM DA and 1.5 mM GSH followed by treatment with 10 mM DTT in indicated samples as described under the “Materials and Methods” section. The protein samples (25 ng) were separated by nonreducing SDS-PAGE followed by Western blot analysis using anti-SSG antibody (top) or anti-CBS antibody (bottom). Molecular masses of protein standards in kDa are shown on the left. The data are representative of at least three independent experiments. IB denotes immunoblotting with either the anti-glutathione (anti-SSG) or anti-CBS antibody. (b) Effect of glutathionylation on CBS activity. Recombinant wild-type CBS (0.5 mg/ml) was incubated with 1 mM DA and 1.5 mM GSH, followed by 10 mM DTT as indicated, and then the samples were dialyzed to remove DA, GSH, and DTT. CBS activity was measured using methylcysteine (10 mM) as a substrate in the 100 mM HEPES buffer, pH 7 .4. The graph represents the relative activity of samples compared with untreated controls and is the mean±SD (n=3), **p<0.01 versus control. The specific activity of the control sample in the methylcysteine assay (using DTNB detection) was 2.0±0.1 μmol methanethiol h−1 mg protein−1. CBS, cystathionine β-synthase; DA, diamide; DTNB, 5,5′-dithiobis-2-nitrobenzoic acid; DTT, dithiothreitol; GSH, glutathione; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.