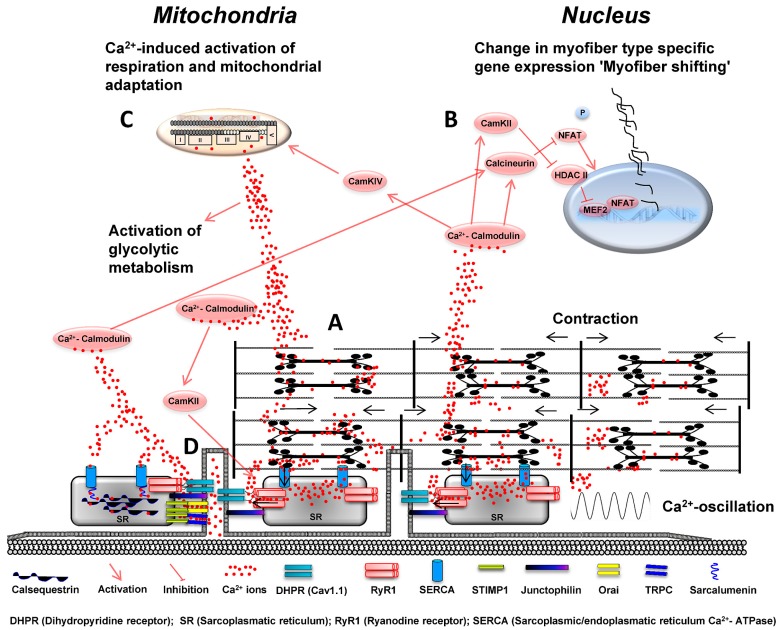
Figure 1.
(A) Voltage-dependent activation of the dihidropyridine receptor (DHPR-Cav1.1) facilitates the release of Ca2+ ions out of the sarcoplasmatic reticulum (SR), which critically regulates skeletal muscle contraction. Reuptake of Ca2+ ions in the SR controls skeletal muscle relaxation and is mainly regulated by ATP-dependent sarcoplasmic/endoplasmic reticulum calcium ATPase pumps (SERCA1/2). Increased neuromuscular activity establishes an oscillating pattern of Ca2+ ion levels and causes elevated sarcoplasmic Ca2+ ion concentrations in the microenvironment of myofibrils; (B) Increasing levels of Ca2+ ions in the sarcoplasm bind to and activate calmodulin (CaM) which regulates activation of calcineurin and calmodulin kinase II and IV. Calmodulin kinase II (CaMKII) contributes to the phosphorylation of ryanodine receptor 1 (RyR1) which increases RyR1 channel activity and open probability. CaMKII further inhibits histone deacetylase II (HDACII) and increases nuclear abundance of myocyte enhancer factor 2 (MEF2). Calcineurin (CaN) dephosphorylates nuclear factor of activated T-cells (NFAT) hereby regulating its nuclear localization. NFAT and MEF2 facilitate the increased expression of “slow genes” coding protein isoforms of the oxidative fiber type; (C) CaMKIV increases the expression of mitochondrial genes, which contributes to mitochondrial adaptation. Free Ca2+ ions also directly stimulate or inhibit Ca2+ release via RyR1 in dependency of their luminal and sarcoplasmic Ca2+ concentration. Ca2+ ions further co-regulate the activation of energy metabolism by activating mitochondrial respiration and increasing the activity of glycolytic enzymes in sarcoplasm; and (D) store-operated calcium entry (SOCE) is regulated by stromal interaction molecule 1 (STIM1) which senses declined Ca2+ ion concentrations in the SR. Interaction of STIMP1 with Orai1 and canonical transient receptor potential channels (TRPC) leads to trans-sarcolemmal Ca2+ influx to increase intracellular Ca2+ levels upon declining Ca2+ content of the SR. Junctophilin maintains junctional triad integrity by overspanning the space between SR and plasma membrane and supports DHPR and RyR1 interaction. Ca2+ uptake and handling is enhanced by sarcalumenin which interacts with SERCA channels and calsequestrin.