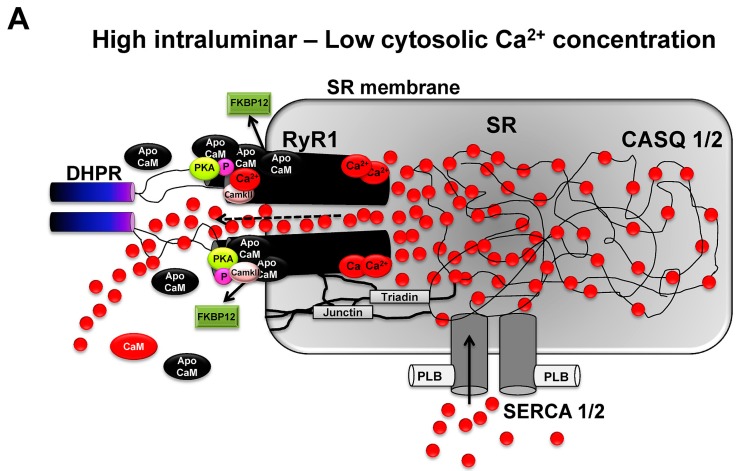
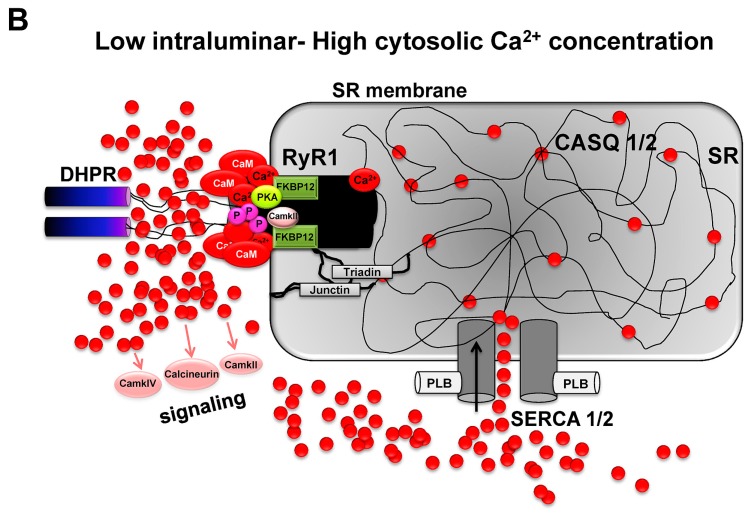
Figure 2.
RyR1 channels induce rapid and high gradients in Ca2+ ion concentration between luminal compartments of the SR and surrounding sarcoplasm of myofbrills. (A) RyR1 opening is primarily controlled by the voltage dependent activation of DHPR which mechanically interacts with RyR1 and regulates channel opening (voltage gating). Further and adjacent RyR1 channels are opened by voltage-independent RyR1-RyR1 interactions (coupled gating) which create locally high Ca2+ ion gradients. On the luminar side of the SR, RyR1 channel opening is supported by the combined mechanical interaction with triadin, junctin and the SR membrane. High Ca2+ ion concentrations in the SR further supports channel open probability. On the sarcoplasmic side and at low Ca2+ concentrations, high amounts of Ca2+ unbound apoCaM supports increased open probability and activity of RyR1 while Ca2+-activated CaM inhibits RyR1. PKA and CaMKII have binding sites on RyR1 subunits and are able to phosphorylate RyR1 and modulate channel activity; and (B) Upon elevation of sarcoplasmic Ca2+ levels due to RyR1 channel opening, decreased Ca2+ ion levels in SR inhibit RyR1 channel activity. Sarcoplasmic Ca2+ levels increase CaM levels which activate CaMKII, IV and calcineurin. CaM inhibits RyR1 channel activity on the sarcoplasmic side of RyR1 while CaMKII binds to and phosphorylates RyR1. Hyperphosphorylation of RyR1 via PKA and CaMKII may lead to increased dissociation of FKBP12, higher open probability of RyR1 channels and decreased contractility of skeletal muscle under resting conditions. Increased SERCA activity facilitates rapid reuptake of Ca2+ ions into the SR.