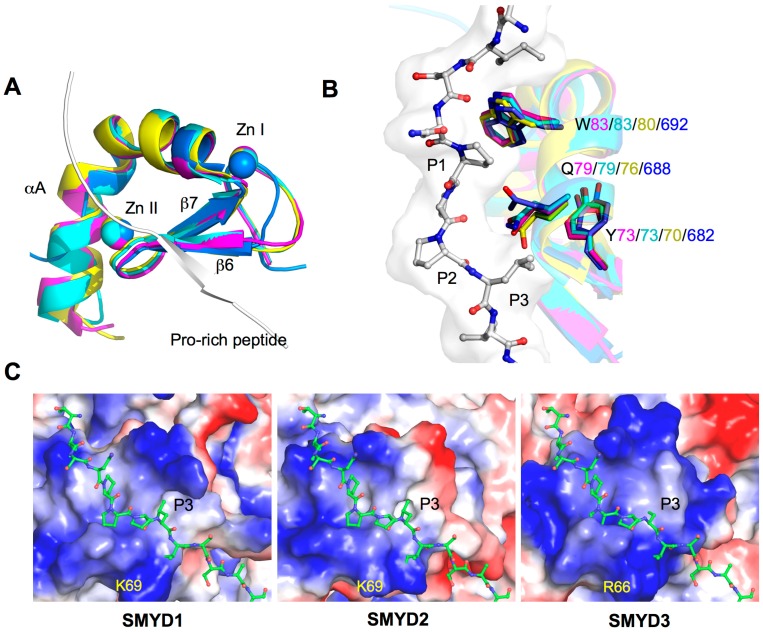
Figure 2.
Structure of MYND domains. (A) Structural superposition of the MYND domains of SMYD and AML1/ETO (PDB code: 2ODD). MYND is represented by ribbon and colored in magenta (SMYD1), cyan (SMYD2), yellow (SMYD3), and blue (AML1/ETO). Proline-rich peptide bound to AML1/ETO is depicted by ribbon; (B) Superposition of the peptide binding pockets. Putative peptide interacting residues are colored according to the scheme in (A). The proline-rich peptide bound to AML1/ETO is depicted by balls-and-sticks; and (C) Surface representation of the MYND domains. Coloring is according to the electrostatic potential: red, white, and blue correspond to negative, neutral, and positive potential, respectively. The vacuum electrostatics/protein contact potential was generated by PyMOL. The proline-rich peptide, represented by balls-and-sticks, is modeled by superposition of the MYND domain of SMYD and AML1/ETO.