Abstract
The acute respiratory distress syndrome (ARDS) is a syndrome of acute respiratory failure associated with severe inflammation and diffuse alveolar damage. Recent studies have demonstrated that the soluble receptor for advanced glycation end products (sRAGE) plays an important role in the pathogenesis of ARDS. The aim of this study was to ascertain whether plasma levels of sRAGE were elevated in ARDS patients compared with appropriate controls. Furthermore, we explored whether plasma levels of sRAGE were related to disease severity, ventilatory parameters and clinical outcome. We prospectively enrolled twenty-two ARDS patients, fourteen ventilated controls and twelve healthy subjects. The Sequential Organ Failure Assessment (SOFA) score was applied to assess illness severity. In addition, ventilator parameters (arterial oxygen tension (PaO2): inspiratory oxygen fraction (FiO2) ratio, arterial carbon dioxide tension (PaCO2), tidal volume and positive end-expiratory pressure (PEEP)) of ARDS patients and ventilated controls were also recorded. Plasma samples were collected within 24 hours and levels of sRAGE were determined using a commercially available sandwich enzyme-linked immunosorbent assay (ELISA) kit. Possible correlation between plasma sRAGE levels and clinical parameters were explored using a simple linear model. Plasma sRAGE levels were significantly elevated in the plasma samples taken from patients with ARDS (1797 ± 383 pg/ml) when compared with both ventilated (650 ± 192 pg/ml, P < 0.01) and healthy (415 ± 178 pg/ml, P < 0.01) controls. Significant correlations were found between plasma sRAGE levels and PaO2:FiO2 ratio (P < 0.05, r=0.36). There was no significant difference in plasma sRAGE levels between survivors and non-survivors (P=0.34). Our results demonstrate that elevated levels of plasma sRAGE may provide a useful marker for ventilated ARDS patients. Furthermore, the relationship between plasma sRAGE levels and PaO2:FiO2 ratio in the ARDS population provides the hypothesis that ventilatory strategy may influence alveolar epithelial damage in ARDS.
Keywords: Acute respiratory distress syndrome, soluble receptor for advanced glycation end products, clinical outcome
Introduction
The acute respiratory distress syndrome (ARDS) is a syndrome of acute respiratory failure associated with severe inflammation and diffuse alveolar damage caused by a variety of injurious insults [1]. ARDS has a high incidence and overall mortality remains high, which brings a substantial impact on public health [1-3]. Disruption of the alveolar epithelial lining is a key feature in the pathophysiology of ARDS, and leads to the development of pulmonary edema and respiratory failure [4].
Recent studies have demonstrated that the receptor for advanced glycation end products (RAGE) plays an important role in the pathogenesis of ARDS, due to the fact that it is abundant in the lung and its expression is primarily located on the basal membranes of alveolar type I epithelial cells [1,5,6]. Soluble RAGE (sRAGE), consisting of the extracellular domain but lacking the transmembrane and cytoplasmic domains of RAGE, has been described in human plasma [7]. Circulating levels of sRAGE have been reported to be associated with many diseases, including type 2 diabetes [8], coronary artery disease [9], and Alzheimer disease [10]. In 2006, Uchida T et al. showed in their study that plasma sRAGE levels in patients with acute lung injury were significantly higher compared with patients with hydrostatic pulmonary edema or healthy volunteers [6].
The aim of this study was to ascertain whether plasma levels of sRAGE were elevated in ARDS compared to appropriate controls in our intensive care unit. Furthermore, we also explored whether plasma levels of sRAGE were related to disease severity, ventilatory strategy and clinical outcome.
Materials and methods
Study patients
We prospectively enrolled twenty-two ARDS patients from our hospital between January 2012 and August 2013. Patients with ARDS participated in this study met the American-European Consensus Conference (AECC) criteria [11] and were included within the first 24 hours of disease onset. The exclusion criteria were age < 18 years, pregnancy, and left ventricular failure [12]. Fourteen mechanically ventilated patients of non-pulmonary disorders and twelve healthy subjects matched for age and sex served as controls.
Clinical data and plasma samples collection
The following clinical data were collected at enrollment: age, gender, aetiology of ARDS. The Sequential Organ Failure Assessment (SOFA) score was also applied to assess illness severity. In addition, ventilator parameters (arterial oxygen tension (PaO2): inspiratory oxygen fraction (FiO2) ratio, arterial carbon dioxide tension (PaCO2), tidal volume and positive end-expiratory pressure (PEEP)) of ARDS patients and ventilated controls were also recorded. Blood were sampled and processed within 24 hours at enrollment. Plasma samples then were stored at -80°C until analysis.
Measurement of plasma sRAGE levels
Plasma levels of sRAGE were determined using a commercially available sandwich enzyme-linked immunosorbent assay (ELISA) kit which was purchased from R&D Systems (Minneapolis, MN, USA).
Statistical analysis
All Statistical analyses were performed using SPSS version 17.0 (SPSS Inc.) and GraphPad Prism version 5.01 (GraphPad Software Inc.). Qualitative data were expressed as numbers or proportions. Proportions between groups were compared using Chi-square test. Variables with numbers were expressed as means ± standard deviation or means ± standard error of the mean (for sRAGE levels) and comparisons between different groups were subjected to Students’ t test or one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test, previously checking the homogeneity of variables. Regressions were computed by using a simple linear model. Differences were considered significant when P < 0.05.
Results
Characteristics of patients
Baseline characteristics and ventilatory parameters of the study population are presented in Table 1. The primary disorders in the studying ARDS patients were pneumonia (n=16), aspiration (n=3), lung contusion (n=1), extra pulmonary sepsis (n=2). There were no differences in baseline characteristics (neither age nor gender) among three groups. As also illustrated in Table 1, PaCO2 levels and PEEP were significantly higher in ARDS compared with the ventilated controls (both P < 0.01). However, PaO2:FiO2 ratios were significantly lower in patients with ARDS (P < 0.01).
Table 1.
Characteristics of the studying population
| Parameters | ARDS patients | Ventilated controls | Healthy controls | P-value (ARDS versus ventilated controls) |
|---|---|---|---|---|
| Number of subjects | 22 | 14 | 12 | |
| Age (years, mean ± SD) | 61 ± 13 | 59 ± 15 | 59 ± 16 | p=0.67 |
| Female sex (n, %) | 9 (41%) | 6 (43%) | 6 (50%) | p=0.91 |
| Cause of ARDS (n, %) | ||||
| Pneumonia | 16 | |||
| Aspiration | 3 | |||
| Lung contusion | 1 | |||
| Extra pulmonary sepsis | 2 | |||
| SOFA score | 6 ± 3 | 7 ± 3 | p=0.34 | |
| PaO2:FiO2 ratio (kPa) | 16.2 ± 3.3 | 32.8 ± 3.5 | p < 0.01 | |
| PaCO2 (kPa) | 8.1 ± 1.0 | 5.9 ± 1.1 | p < 0.01 | |
| Tidal volume (mL/kg predicted weight) | 9.1 ± 2.2 | 9.5 ± 2 | p=0.58 | |
| PEEP (cmH2O) | 9.4 ± 2.0 | 6.2 ± 1.4 | p < 0.01 | |
| sRAGE levels (pg/ml, mean ± SEM) | 1797 ± 383 | 650 ± 192 | 415 ± 178 | p < 0.01 |
ARDS, acute respiratory distress syndrome; SOFA, Sequential Organ Failure Assessment; PaO2, arterial oxygen tension; FiO2, inspiratory oxygen fraction; PaCO2, arterial carbon dioxide tension; PEEP, positive end-expiratory pressure; sRAGE, soluble receptor for advanced glycation end products; SD, standard deviation; SEM, standard error of the mean.
Correlation between plasma sRAGE levels and clinical parameters
Plasma sRAGE levels were significantly elevated in the plasma samples taken from patients with ARDS (1797 ± 383 pg/ml) when compared with both ventilated (650 ± 192 pg/ml, P < 0.01) and healthy (415 ± 178 pg/ml, P < 0.01) controls (Figure 1). We next explored the possible correlation between plasma sRAGE levels and clinical parameters using a simple linear model. As shown in Table 2, there was no correlation between plasma sRAGE levels and global illness severity as assessed by SOFA score (P=0.38, r=0.21). However, significant correlations were found between plasma sRAGE levels and PaO2:FiO2 ratio (P < 0.05, r=0.36) in patients with ARDS.
Figure 1.
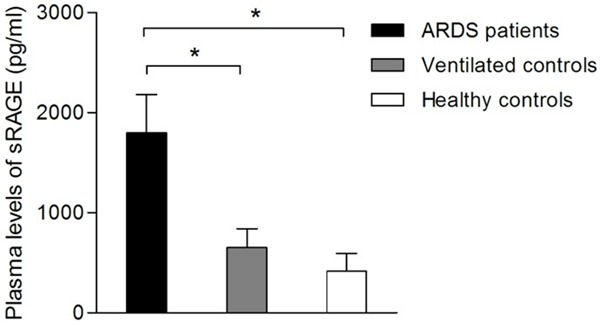
Plasma soluble receptor for advanced glycation end products (sRAGE) levels in patients with acute respiratory distress syndrome (ARDS), ventilated controls and healthy controls. Data are presented as means ± SEM (*P < 0.05).
Table 2.
Correlation of plasma sRAGE levels and clinical parameters
| Parameters | Correlation with plasma sRAGE (pg/ml) |
|---|---|
| SOFA score | P=0.38, r=0.21 |
| PaO2:FiO2 ratio (kPa) | P < 0.05, r=0.36 |
| PaCO2 (kPa) | P=0.78, r=0.06 |
| Tidal volume (mL/kg predicted weight) | P=0.08, r=0.39 |
| PEEP (cmH2O) | P=0.18, r=0.33 |
sRAGE, soluble receptor for advanced glycation end products; SOFA, Sequential Organ Failure Assessment; PaO2, arterial oxygen tension; FiO2, inspiratory oxygen fraction; PaCO2, arterial carbon dioxide tension; PEEP, positive end-expiratory pressure.
Plasma sRAGE levels and clinical outcome
To further determine whether plasma sRAGE levels were associated with the outcome of ARDS, we analyzed the data from survivors and nonsurvivors of ARDS patients. As shown in Table 3, survivors and non-survivors displayed significant differences in SOFA score and tidal volume, which were both higher in the non surviving group, both P < 0.01). PaO2:FiO2 ratios were significantly lower in the non surviving group (P < 0.01). However, there was no significant difference in plasma sRAGE levels between survivors and non survivors (Figure 2, P=0.34), as well as PaCO2 and PEEP (P=0.05 and 0.09, respectively).
Table 3.
Characteristics, ventilatory parameters and sRAGE levels for survivors and non-survivors of ARDS patients
| Parameter | ARDS survivors | ARDS non-survivors | P-value |
|---|---|---|---|
| Number of subjects | 12 | 10 | |
| Age (years, mean ± SD) | 60 ± 12 | 62 ± 14 | P=0.73 |
| Female sex (n, %) | 9 (41%) | 6 (43%) | P=0.91 |
| SOFA score | 4 ± 2 | 9 ± 3 | P < 0.01 |
| PaO2:FiO2 ratio (kPa) | 19.6 ± 3.5 | 12.2 ± 3.1 | P < 0.01 |
| PaCO2 (kPa) | 8.5 ± 1.2 | 7.6 ± 0.8 | P=0.05 |
| Tidal volume (mL/kg predicted weight) | 8.1 ± 2.0 | 10.9 ± 2.4 | P < 0.01 |
| PEEP (cmH2O) | 8.7 ± 2.1 | 10.2 ± 1.9 | P=0.09 |
| sRAGE levels (pg/ml, mean ± SEM) | 1548 ± 431 | 2096 ± 317 | P=0.34 |
sRAGE, soluble receptor for advanced glycation end products; ARDS, acute respiratory distress syndrome; SOFA, Sequential Organ Failure Assessment; PaO2, arterial oxygen tension; FiO2, inspiratory oxygen fraction; PaCO2, arterial carbon dioxide tension; PEEP, positive end-expiratory pressure; SD, standard deviation; SEM, standard error of the mean.
Figure 2.
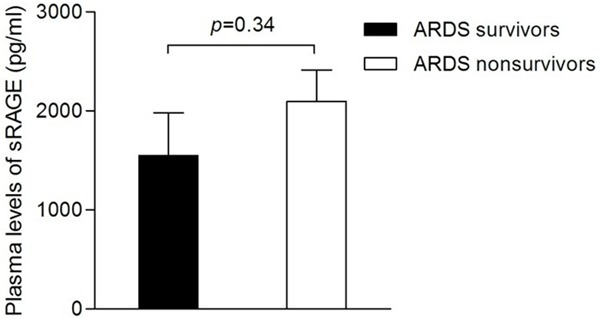
Plasma soluble receptor for advanced glycation end products (sRAGE) levels in survivors and nonsurvivors of ARDS patients. Data are presented as means ± SEM.
Discussion
In this study, our results demonstrate that plasma levels of sRAGE from patients with ARDS were elevated when compared with ventilated and healthy controls. Furthermore, significant correlations were detected for the first time between plasma sRAGE levels and PaO2:FiO2 ratio in the ARDS population. However, there was no relationship between sRAGE and global illness severity as assessed by SOFA score in our study.
RAGE is abundantly expressed in alveolar type I epithelium in the lung, and its soluble isoform (sRAGE) is released into the plasma when lung tissue is injured [4,13,14]. The precise mechanism of its release into plasma has not been clearly elucidated. However, animal studies suggested that sRAGE was shed from alveolar type I epithelial cells by proteases [6]. Previous studies have already shown that plasma sRAGE were higher in ARDS patients compared with controls [4,6,15]. Our first results were in conformity with these findings, which suggests that increased plasma levels of sRAGE in patients with ARDS reflect the pathophysiology of lung injury.
We next explored possible correlation between plasma sRAGE levels and clinical parameters such as SOFA score and ventilatory parameters. Significant correlations were found between plasma sRAGE levels and PaO2:FiO2 ratio in patients with ARDS. Previous study has reported that patients with high plasma sRAGE were those who benefited most from lung protective ventilation [16]. Our results also indicated that ventilatory strategy may relate to plasma sRAGE levels, which is a marker of alveolar epithelial damage in ARDS.
Finally, we analyzed whether plasma sRAGE levels were associated with clinical outcome of ARDS patients. Although plasma sRAGE levels were elevated in nonsurvivors compared with survivors of ARDS patients, this difference was not significant in our study. Previous studies also presented inconsistent results. Mauri T et al in 2010 found this difference was not statistically significant [15], whereas Nakamura T et al in 2011 demonstrated significant difference [4]. In our limited opinion, some reasons can explain this disparity. First, numbers of patients enrolled in our study and those two studies were relatively small and may not be adequate to provide statistically meaningful data. Second, as previously reported, different isoforms of sRAGE have been described and different ELISA techniques may detect different isoforms [6,13]. Third, plasma samples used in our study had been stored for several days or months at -80°C, as reported in previous studies [16,17]; whether this extended storage has any effects on plasma sRAGE levels remains unknown.
In conclusion, our study demonstrates that elevated levels of plasma sRAGE may provide a useful marker for ventilated ARDS patients. Furthermore, the relationship between plasma sRAGE levels and PaO2:FiO2 ratio in the ARDS population provides the hypothesis that ventilatory strategy may influence alveolar epithelial damage in ARDS.
Acknowledgements
The authors would like to thank all of the nurses in our intensive care unit for the help of blood sample collection.
Disclosure of conflict of interest
None.
References
- 1.Guo WA, Knight PR, Raghavendran K. The receptor for advanced glycation end products and acute lung injury/acute respiratory distress syndrome. Intensive Care Med. 2012;38:1588–1598. doi: 10.1007/s00134-012-2624-y. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23:243–252. doi: 10.1089/jamp.2009.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Maeda S, Yamagishi S. Increased levels of soluble receptor for advanced glycation end products (sRAGE) and high mobility group box 1 (HMGB1) are associated with death in patients with acute respiratory distress syndrome. Clin Biochem. 2011;44:601–604. doi: 10.1016/j.clinbiochem.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Shirasawa M, Fujiwara N, Hirabayashi S, Ohno H, Iida J, Makita K, Hata Y. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells. 2004;9:165–174. doi: 10.1111/j.1356-9597.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- 6.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173:1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jabaudon M, Futier E, Roszyk L, Chalus E, Guerin R, Petit A, Mrozek S, Perbet S, Cayot-Constantin S, Chartier C, Sapin V, Bazin JE, Constantin JM. Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients. Crit Care Med. 2011;39:480–488. doi: 10.1097/CCM.0b013e318206b3ca. [DOI] [PubMed] [Google Scholar]
- 8.Tan KC, Shiu SW, Chow WS, Leng L, Bucala R, Betteridge DJ. Association between serum levels of soluble receptor for advanced glycation end products and circulating advanced glycation end products in type 2 diabetes. Diabetologia. 2006;49:2756–2762. doi: 10.1007/s00125-006-0394-1. [DOI] [PubMed] [Google Scholar]
- 9.Falcone C, Emanuele E, D’Angelo A, Buzzi MP, Belvito C, Cuccia M, Geroldi D. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol. 2005;25:1032–1037. doi: 10.1161/01.ATV.0000160342.20342.00. [DOI] [PubMed] [Google Scholar]
- 10.Emanuele E, D’Angelo A, Tomaino C, Binetti G, Ghidoni R, Politi P, Bernardi L, Maletta R, Bruni AC, Geroldi D. Circulating levels of soluble receptor for advanced glycation end products in Alzheimer disease and vascular dementia. Arch Neurol. 2005;62:1734–1736. doi: 10.1001/archneur.62.11.1734. [DOI] [PubMed] [Google Scholar]
- 11.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 12.Guervilly C, Lacroix R, Forel JM, Roch A, Camoin-Jau L, Papazian L, Dignat-George F. High levels of circulating leukocyte microparticles are associated with better outcome in acute respiratory distress syndrome. Critical Care. 2011;15:R31. doi: 10.1186/cc9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uchida T, Ohno N, Asahara M, Yamada Y, Yamaguchi O, Tomita M, Makita K. Soluble isoform of the receptor for advanced glycation end products as a biomarker for postoperative respiratory failure after cardiac surgery. PLoS One. 2013;8:e70200. doi: 10.1371/journal.pone.0070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Determann RM, Wolthuis EK, Choi G, Bresser P, Bernard A, Lutter R, Schultz MJ. Lung epithelial injury markers are not influenced by use of lower tidal volumes during elective surgery in patients without preexisting lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;294:L344–350. doi: 10.1152/ajplung.00268.2007. [DOI] [PubMed] [Google Scholar]
- 15.Mauri T, Masson S, Pradella A, Bellani G, Coppadoro A, Bombino M, Valentino S, Patroniti N, Mantovani A, Pesenti A, Latini R. Elevated plasma and alveolar levels of soluble receptor for advanced glycation endproducts are associated with severity of lung dysfunction in ARDS patients. Tohoku J Exp Med. 2010;222:105–112. doi: 10.1620/tjem.222.105. [DOI] [PubMed] [Google Scholar]
- 16.Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, Matthay MA, Network NA. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax. 2008;63:1083–1089. doi: 10.1136/thx.2008.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jabaudon M, Perbet S, Pereira B, Soummer A, Roszyk L, Guerin R, Futier E, Lu Q, Bazin JE, Sapin V, Rouby JJ, Constantin JM. Plasma levels of sRAGE, loss of aeration and weaning failure in ICU patients: a prospective observational multicenter study. PLoS One. 2013;8:e64083. doi: 10.1371/journal.pone.0064083. [DOI] [PMC free article] [PubMed] [Google Scholar]


