Figure.
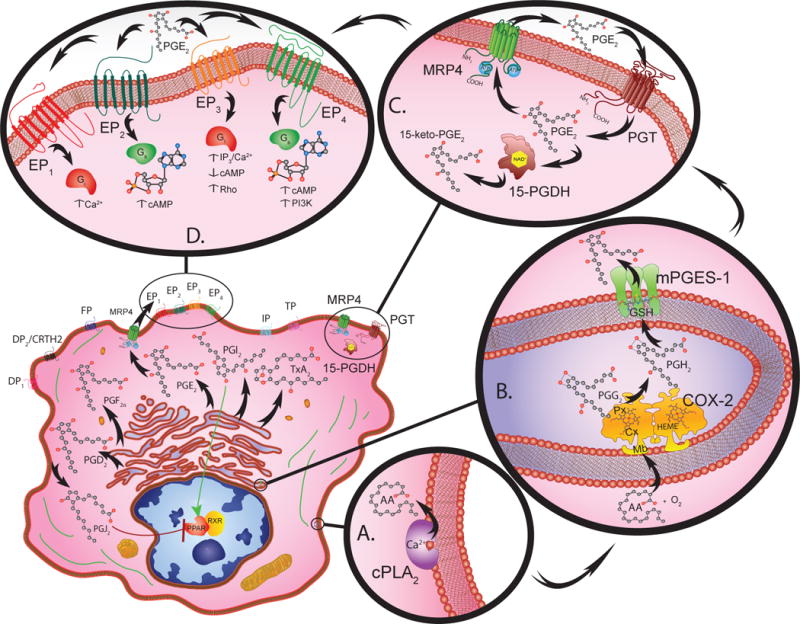
Eicosanoid biosynthesis, metabolism, and signal transduction requires the cooperative interaction between multiple compartments within a given cell (lower left). Cytosolic phospholipase A2 (PLA2) catalyzes the calcium dependent release of arachidonic acid (AA) from membrane phospholipids (A.).
Free AA serves as a substrate for COX-2 (72kDa) monomer subunits that form functional homodimer complexes (B.). Each monomer has a membrane-binding domain (Mb) that anchors the protein into the membrane of the endoplasmic reticulum or nuclear envelope. The catalytic domain contains cyclooxygenase (Cx) and peroxidase (Px) active sites that are organized on either side of a heme (HEME) prosthetic group. The cyclooxygenase site converts arachidonic acid to hydroperoxy-endoperoxide prostaglandin G2 (PGG2) through the addition of two O2 molecules. The peroxidase site then reduces PGG2 to PGH2.
Once PGH2 is generated, various synthase molecules convert it to bioactive PGs. Of these synthases, mPGES-1 is primarily responsible for increasing the PGE2 levels that promote inflammation, and tumorigenesis (B.). mPEGS-1 exists as a 16kDa monomer that forms active homotrimer complexes by interacting with glutathione (GSH) in perinuclear or endoplasmic reticulum membranes.
Prostanoids are transported into the extracellular microenvironment by specific multidrug resistance associated proteins (MRP). These MRP molecules contain a 12-membrane spanning domain structure that contains two cytosolic ATP-binding/hydrolysis sites. Among these transmembrane molecules, MRP4 is a 160kDa protein that acts as the primary transporter for PGs (C.).
The PG receptors, DP1, DP2, EP1-4, FP, IP and TP are G-protein coupled receptors classified according to their ligand specificity (D.). There are four EP receptors that rely on G-stimulatory (Gs) or G-inhibitory (Gi) proteins to activate second messengers such as cAMP, Ca2+, and inositol phosphates to initiate downstream signaling. More specifically, EP1 regulates Ca2+ flux; EP2 and EP4 both increase cAMP levels; whereas EP3 decreases cAMP, increases IP3/Ca2+, and activates Rho. Peroxisome proliferator-activated receptors (PPAR) also bind PGs and complex with retinoic X receptors (RXR) to initiate gene transcription.
The catabolism of PG involves two-steps, uptake and inactivation (C.). PGs are taken up by a 12 transmembrane domain glycoprotein known as a PG transporter (PGT). After PGE2 is transported across the plasma membrane it is enzymatically catabolized by NAD+ dependent 15-hydroxyprostaglandin dehydrogenase (15-PGDH) causing inactivation. The NAD+-15-PGDH monomers (29 kDa) dimerize into enzymatically active complexes, which form 15-keto inacitve metabolites.
