Table 1. . Summary of most frequent molecular and clinical characteristics of pediatric high-grade gliomas.
| Mutations/cytogenetics | H3F3AmutK27 | H3F3AmutG34 | RTK I (PDGFRA ampl.) | SETD2 | ACVR1 | BRAFV600E | NTRK |
|---|---|---|---|---|---|---|---|
| Age distribution (years) | 5–29 | Median: 20 | Median: 11.4 | >12 | 5–9 | 6.5–20 | <3 |
| Tumor location |
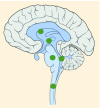 23–43% |
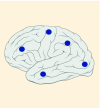 12–14% |
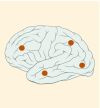 8–39% |
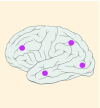 15% |
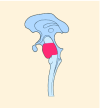 20–22% |
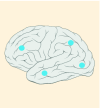 10% |
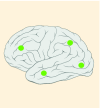 40% |
| Therapeutic Significance | None | None | PDGFR inhibitors | Temozolomide resistance | Potential (ALK inhibitors) | BRAF inhibitors | Potential (NTRK inhibitors) |
K27M tumors are found in the midline areas (thalamus, pons, brainstem and spine), they tend to occur at a median age of 10.5 years. G34R/V tumors are found in the cerebral hemispheres, usually occur in adolescents (median age: 18 years) [6,18,20,36,37]. Focal amplifications in PDGFRA are mainly associated with diffuse intrinsic pontine gliomas, the incidence might be overestimated because of the suspected overexpression of PDGFRA in radiation-exposed tumors [6,8,20,68], and with a subset of hemispheric tumors. The median age of patients having tumors with PDGFRA alterations is 11.4 years [6]. Mutations in SETD2 occur exclusively in the cerebral hemispheres in patients older than 12 years [6,40,71]. A recent study found an association between SETD2 mutations and defective DNA mismatch repair through microsatellite instability [41]. Mutations in ACVR1 are reported exclusively in diffuse intrinsic pontine gliomas and are found in 20–22% of these patients, most of which are in pretreated samples [22,58–60]. BRAF V600E point mutations in pediatric high-grade gliomas occur in young adults and most of the tumors are located in the cerebral hemispheres [6]. NTRK fusions in NTRK1, NTRK2 and NTRK3 genes are found in the cerebral hemispheres of patients younger than 3 years of age [60].
