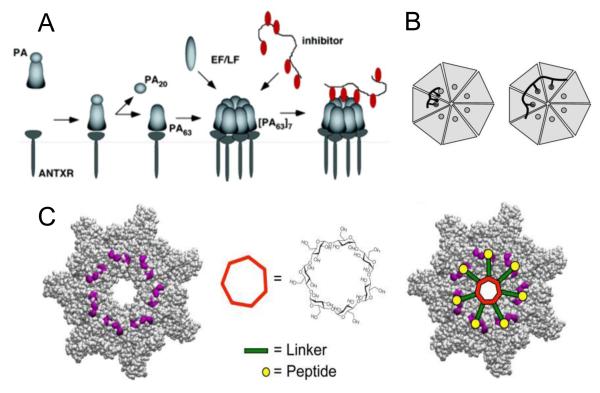
Figure 3.
Inhibition of toxin assembly by polyvalent inhibitors. (A) After being cleaved by extracellular furin protease to a 63-kDa form, PA63 oligomerizes and binds either EF/LF or a peptide-based inhibitor (74). (B) Design of polyvalent inhibitors with control over the molecular weight and ligand spacing (76). The linear polyvalent inhibitors displaying peptides (black ovals) are shown bound to the PA63 heptamer at the peptide-binding sites (circles). The spacing between peptides on the linear scaffold is either too short (left panel) or sufficient (right panel) to allow polyvalent interactions. (C) Structure-based design of the heptavalent anthrax toxin inhibitors (80). Left: The structure of the LF-binding face of heptameric PA63. Residues 184, 187, 197, and 200, which form part of the peptide-binding site, are shown in purple. Middle: The structure of 7-fold symmetrical β-cyclodextrin, which was used as a scaffold for a heptameric inhibitor. Right: A scheme illustrating the binding of the heptavalent inhibitor, synthesized by the attachment of seven inhibitory peptides to the β-cyclodextrin via an appropriate PEG linker, to heptameric PA63. Reprinted with permission from refs (74, 76, 80). Copyright 2005, 2006, 2011. American Chemical Society.