Highlights
-
•
We modelled the surface water salinity in the Baltic from the 1960s to 2100.
-
•
We studied possible changes in distribution areas of predominant plant, invertebrate and fish species.
-
•
The results suggest a critical shift in the salinity range 5–7, which is a bottleneck for both marine and freshwater species distribution and diversity.
-
•
This foreseen salinity change is likely to have large impacts on marine ecology, it́s monitoring, modelling as well as fisheries.
Keywords: Environmental monitoring and conservation, Species richness, Foundation species, Zoogeographic distribution, Global environmental change
Abstract
Substantial ecological changes occurred in the 1970s in the Northern Baltic during a temporary period of low salinity (S). This period was preceded by an episodic increase in the rainfall over the Baltic Sea watershed area. Several climate models, both global and regional, project an increase in the runoff of the Northern latitudes due to proceeding climate change. The aim of this study is to model, firstly, the effects on Baltic Sea salinity of increased runoff due to projected global change and, secondly, the effects of salinity change on the distribution of marine species. The results suggest a critical shift in the S range 5–7, which is a threshold for both freshwater and marine species distributions and diversity. We discuss several topics emphasizing future monitoring, modelling, and fisheries research. Environmental monitoring and modelling are investigated because the developing alternative ecosystems do not necessarily show the same relations to environment quality factors as the retiring ones. An important corollary is that the observed and modelled S changes considered together with species’ ranges indicate what may appear under a future climate. Consequences could include a shift in distribution areas of marine benthic foundation species and some 40–50 other species, affiliated to these. This change would extend over hundreds of kilometres, in the Baltic Sea and the adjacent North Sea areas. Potential cascading effects, in coastal ecology, fish ecology and fisheries would be extensive, and point out the necessity to develop further the “ecosystem approach in the environmental monitoring”.
1. Introduction
Low salinity (S) forms a threshold for marine and freshwater species distributions in river mouths, estuaries, fjords, lagoons, and coastal seas. A direct control of marine species distributions, through osmoregulation, was earlier focused on in marine biological research, which included seminal studies in the Baltic Sea area dating back to the 1930s. This issue was later updated by Remane and Schlieper (1971) . The regulating effect of S on invertebrates is summarized by Kinne, 1964, Kinne, 1971. The anticipated climate change in the northern coastal areas brings S back into focus. Regional projections concerning global change concentrate on the effects of increased temperature and eutrophication (BACC, 2008, Neumann, 2010, Philippart et al., 2011), however, they also point out a decrease of S in northern coastal areas (Meier et al., 2006). BACC (2008) reviews pelagic fish, deep water benthos, and plankton populations in relation to recent S and demonstrate the profound effect of S changes in the Baltic Sea pelagic and deep water biota. However, there are several reasons to focus on the sea surface S (SSS). The shallow bottom and littoral habitats are directly affected by changes in surface S. The surface layer is ecologically and economically crucial since all primary production takes place there and the secondary production also is greatly dependent of this zone.
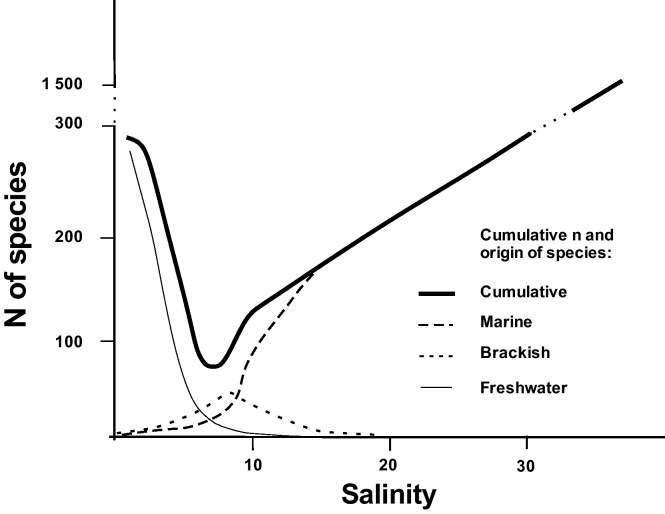
Freshwater runoff and inflows of North Sea water through the Danish Straits control the S levels and biodiversity of the Baltic Sea (Segerstråle, 1957, Järvekülg, 1979, Hänninen et al., 2000; a recent review by BACC, 2008) rendering it a suitable environment for demonstrating the biogeographic aspects of S regulation. The importance of the regulation may be exemplified by its large taxonomic coverage. For each S isoline value may a distinct natural community be defined due to species specific S preferences (Segerstråle, 1957, Järvekülg, 1979, Furman et al., 2001). Thus, the Baltic Sea S gradient limits the distribution of marine species towards the Bothnian Bay in the north and the Gulf of Finland in the east. S of less than three in the northernmost Bay of Bothnia is too low for most marine species, of which reproducing populations are found only of: one Lamellibranchiate, the Baltic tellin (Macoma balthica); one barnacle (Balanus improvisus); and one macroscopic alga, the wrack (Fucus radicans) (Järvekülg, 1979, Bergström et al., 2005). Further south in the Bothnian Sea the Decapods, Palaemon adspersus, Crangon crangon, and the Polychaete Fabricia sabella, are found above salinities of five to six (Järvekülg, 1979). In the Kattegat with S above 25, more marine species start to occur, such as Echinodermata (the starfish, Asterias rubens) and the shore crab (Carcinus moenas). The lowest number of species is found in the S range between five and seven. This range is often called the range of critical S, “horohalinicum” (Kinne, 1971). Next to the lower end of this range, the decrease in the number of fresh water species is highest, while in its upper end, around a S of seven, the decrease of marine species is steepest (Fig. 1).
Fig. 1.
Critical salinity, “horohalinicum” (modified after Remane and Schlieper, 1971), is the range of salinity from approximately 5–7, where both the freshwater (on the left) and marine species (on the right) number declines the steepest in the Baltic Sea. Currently, a total of 6065 species exist in the Baltic Sea (incl. Kattegat). The most numerous are phytoplankton, macrozoobenthos, and zooplankton (at least 1700, 1476, and 1190 species, respectively) (Ojaveer et al., 2010).
In this study, we firstly examine the hydrographic and biological data, reviewing a selected period (from 1960s up to present) when the S of the Baltic Sea was temporarily dropped due to increased runoff (Hänninen and Vuorinen, 2011). Secondly, model projections for future surface water S are presented. Thirdly, possible consequences of S changes are projected in terms of distribution areas of shallow water plant and animal species. Finally, we discuss environmental monitoring, modelling, and fisheries in relation to our projections in order to propose research areas and topics that deserve more attention. As the littoral area is a central environment in harboring species diversity in the Baltic Sea we focus our discussion only on selected, predominant, littoral species. For practical reasons, we focus on distribution of marine species.
2. Review of changes in hydrography and their ecological consequences in the 1970s
Since the 1950s there have been periods of high and low S and precipitation as well as episodic inflows of North Sea water into the Baltic Sea. The observation that species may change their distribution, in concert with S fluctuations, was discussed after a large inflow of highly saline North Sea water into the Baltic Sea in 1951 (Segerstråle, 1969), and again together with decreasing salinities in the 1980s (Vuorinen et al., 1998 Leppäkoski et al., 1999).
For this study, a period with increased precipitation and decreased S, from 1960 up to the present day, was selected. Yearly isohalines of the layer from 0 to 25 m and the percentage of surface area of the Baltic Sea with a S of less than seven, were constructed. In the 1970’s, S was high, with 7 psu on the S coast of Finland (60° N). Since then S has decreased and the 7 psu isopleth lies now between 56 and 58° N. During late 1970s, the area with S of less than seven thus increased in a considerable way in the northern Baltic proper (Fig. 2).
Fig. 2.
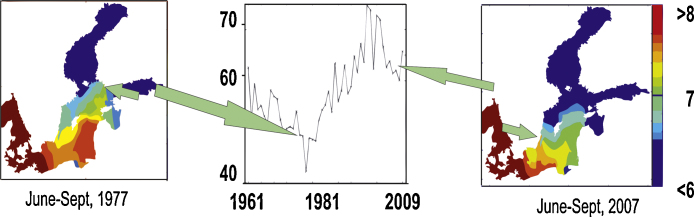
Percentage of area with sea surface salinity less than 7 in the Baltic Sea during the years 1961–2009 (middle panel) and the Baltic Sea surface water (0–25 m) salinities during the years 1977 (left panel) and 2007 (right panel). Source of data: ICES Dataset on Ocean Hydrography (The International Council for the Exploration of the Sea Copenhagen. 2011). SSS of 7 marked with arrows. Salinity gradients above 8 in the Danish Sounds and below 6 in the Bay of Bothnia and Gulf of Finland are not shown.
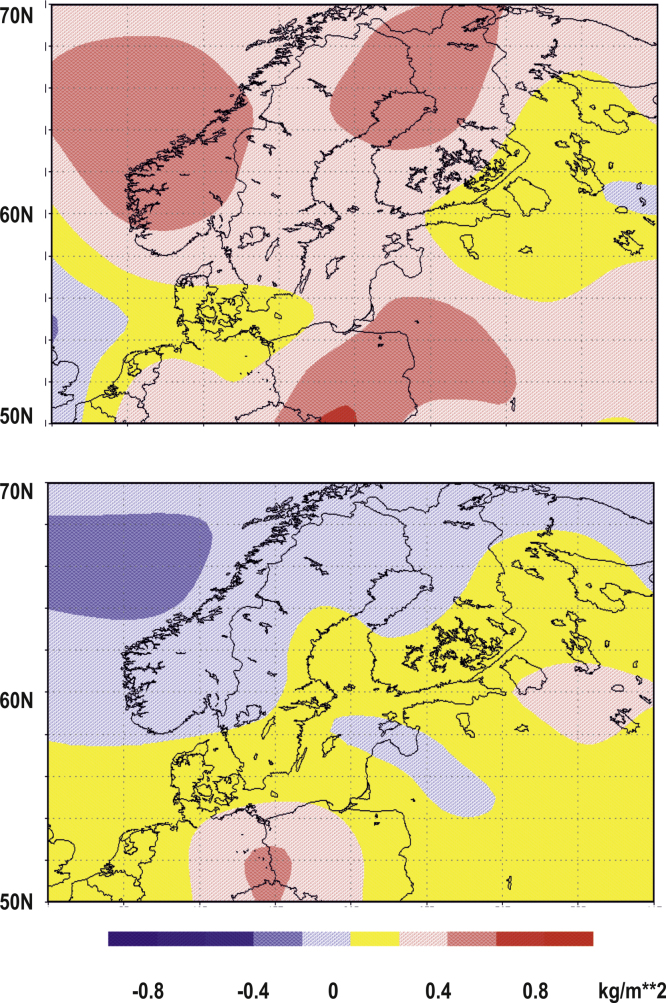
A profound change of SSS can be traced down to increased precipitation and runoff (Hänninen et al., 2000, Hänninen et al., 2000). Using NCEP/NCAR reanalysis data (Kalnay et al., 1996), composites of annual mean anomalies of precipitation rate were constructed for the periods 1960–1975 and 1976–1990. The first period (1960–1975) was characterized by two positive anomalies in precipitation in the Baltic Sea catchment. These occurred between 60° and 70° N and around 50°–56° N (Fig. 3, upper panel). During this period, a stronger than normal runoff prevailed in relatively small northern pristine rivers (The Nordic rivers: Tornionjoki and Kemijoki) and in the, southern Baltic rivers (rivers: Oder and Vistula) (Fig. 4). The two positive anomalies were separated by a band of approximately zero anomalies, which also covered the single largest source of freshwater to the Baltic Sea the Neva runoff area (see Fig. 4). During the period from 1976 to 1990, the zonally oriented band tilted to a NW–SE direction and positive anomalies appeared in most of the catchment area, which spread in the south and east over all major Baltic Sea rivers (rivers Oder, Vistula, Neman, Daugava, Narva and Neva (Fig. 3 lower panel). This resulted in a higher net input of rain into the Baltic Sea during the later period, which was followed by a decrease in the SSS, shifting the horohalinicum southwards and shifting the S of seven approximately to the latitude south of the Island of Gotland (Fig. 2).
Fig. 3.
The composite of the annual mean anomalies of precipitation rate for the period, 1960–1975 (upper panel) and for the period, 1976–1990 (lower panel) in the Baltic Sea watershed compared to the climatic mean during the 1981–2010 reference period. The figure shows a much higher precipitation over the Baltic Sea catchment area during the period 1960–1975 compared to recent data.
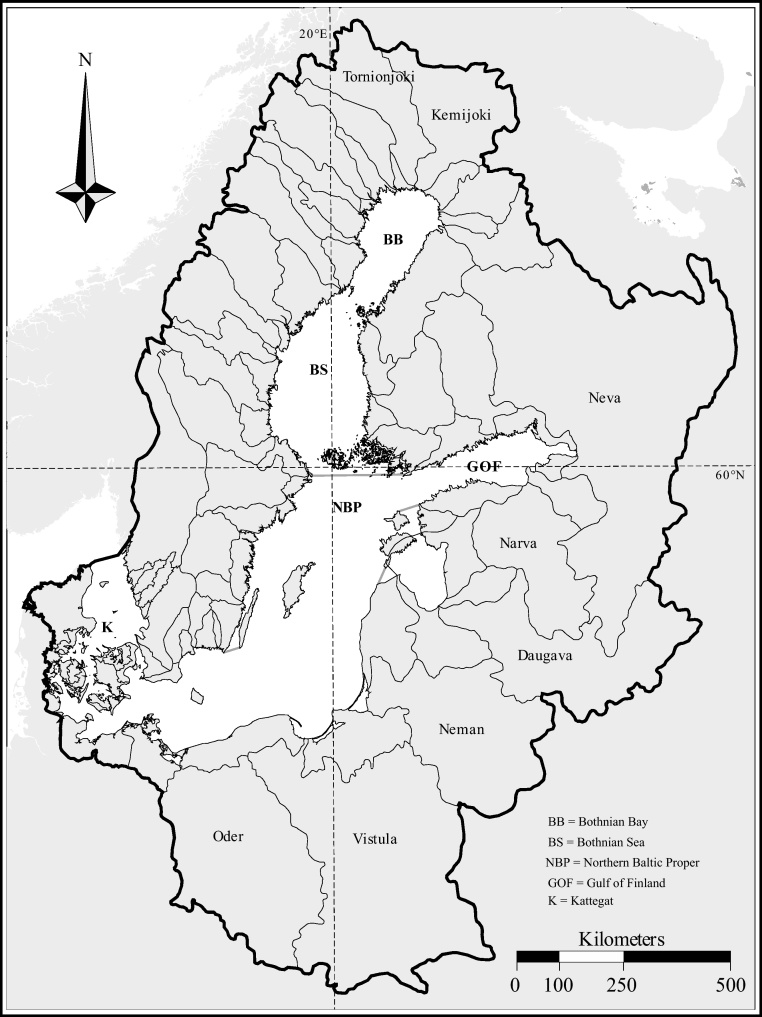
Fig. 4.
Runoff areas of the Baltic Sea rivers and locations mentioned in the text.
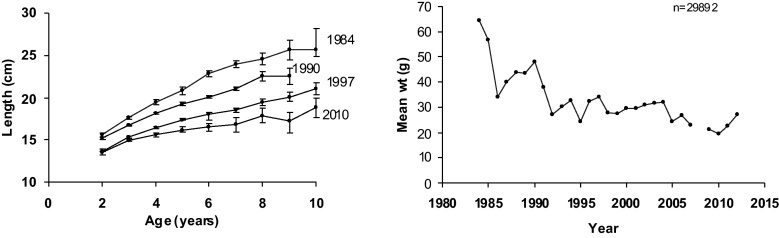
After the 1970s, several marine species covering a variety of taxa have decreased in abundance, distribution area, or suffered from a decreased growth and reproduction, especially in the northern Baltic. Such changes include a decrease in the numbers of large marine Copepoda, especially Pseudocalanus sp. (Ojaveer et al., 1998 Vuorinen et al., 1998) and a marine Ctenophore species Mertensia ovum (Lehtiniemi et al., 2013) as well as in the distribution area of several benthic species (BACC, 2008). Since these are marine species, low salinity imposes increasing osmoregulation costs. Attributing changes to S changes is a reasonable hypothesis. Low S, itself, does not necessarily prevent a species occurring or migrating back and forth in a certain area, but restriction usually comes during reproduction. For instance, in fish, low S can prevent the activation of sperm cells, in which case, fertilization of eggs fails partly or totally. In pelagic spawners, low S reduces the buoyancy of fertilized eggs, which then sink to deeper water layers, where oxygen conditions may turn unfavorable for embryonic development, etc. (Table 1). One of the best known cases may be the Baltic Sea cod, whose reproduction is inhibited in the northern parts of the sea because of a combination of low surface water S and a deep water anoxia after the 1970s (BACC, 2008). A typical case is the decline of growth of the Baltic herring (Fig. 5), which is interpreted as a combination of two simultaneous processes, both of which are ultimately caused by low salinity: the decreased number of large planktonic copepods and an increased food competition among the clupeids (Möllmann et al., 2005 Casini et al., 2006, Casini et al., 2010). The areas first and worst affected were in the northern Baltic proper (e.g. plankton studies by Vuorinen et al., 1998 Ojaveer et al., 1998), which is receiving the largest impact of freshwater runoff from the Neva river watershed, while the southern areas at that time retained a salinity high enough for the sustained normal growth of the Baltic herring (Rajasilta et al., 1999).
Table 1.
Minimum salinity for successful reproduction of some marine species in the Baltic Sea as suggested by experimental studies and/or field observations. Potential threshold phases of reproduction are shown in parenthesis (SA: activation of spermatozoa; F: fertilization; ED: embryonic development; NBE: neutral buoyancy of eggs).
| Taxon | S | Authors |
|---|---|---|
| Angiosperms | ||
| Eelgrass (Zostera marina) | ≥5 | Järvekülg (1979) |
| Algae | ||
| Wrack (Fucus radicans) | 3–5 | Bergström et al. (2005) |
| Echinodermata | ||
| Starfish (Asterias rubens) | 7 | BACC (2008) |
| Ctenophora | ||
| Mertensia ovum | 5.5 | Viherluoto et al. (2013) |
| Moon jellyfish (Aurelia aurita) | ≥5 | Järvekülg (1979) |
| Cnidaria | ||
| Laomedea lovéni | 6.3–7.4 | – |
| Laomedea longissima | 7.6–7.7 | – |
| Laomedea flexuosa | 5.1–7.4 | – |
| Mollusca | ||
| Alderia modesta | ≥5 | Seelemann (1967) |
| Limapontia capitata | 5 | Järvekülg (1979) |
| Hydrobia ulvae | 2.5 | – |
| Littorina littorea | ∼7 | – |
| Common mussel (Mytilus edulis) | 4–5 | Kautsky et al.(1992) |
| Annelida | ||
| Manayunkia estuarina | ≥5.3 | Järvekülg (1979) |
| Fabricia sabella | ≥5.2 | – |
| Paranais frici | ≥5.9 | – |
| Crustacea | ||
| Copepoda | ||
| Pseudocalanus acuspes nauplii | 7.1 | Renz and Hirche (2006) |
| Amphipoda | ||
| Gammarus salinus | 5.9 | Järvekülg (1979) |
| G. zaddachii | 5.7 | – |
| G. locusta | 5.7 | – |
| Pontoporeia femorata | 5.6 | – |
| Galliopius laeviusculus | 5.9 | – |
| Mysidacea | ||
| Mysis relicta | ||
| M. mixta | 5.3 | – |
| Praunus flexuosus | 5.2 | – |
| P. inermis | 5.0 | – |
| Decapoda | 5.0 | – |
| Palaemon adspersus | 5.2 | – |
| Crangon crangon | 5.1 | – |
| Carcinus maenas | 7 | – |
| Pisces | ||
| Cod (Gadus morhua) | 11–12 (SA) | Nissling and Westin (1997) |
| 14–15 (NBE) | – | |
| Sprat (Sprattus sprattus) | 7–8 (NBE) | Nissling et al. (2003) |
| Karaseva and Ivanovich (2010) | ||
| Herring (Clupea harengus membras) | 3–4 (F, ED) | Ojaveer (1981) |
| Klinkhardt (1984) | ||
| Griffin et al. (1998) | ||
| Flounder (Pleuronectes flesus) | ≥6 | Nissling et al. (2002) |
| Turbot (Scophthalmus maximus) | ≥7 | Nissling et al. (2006) |
| Dab (Limanda limanda) | 18–19 (NBE) | Nissling et al. (2002) |
| Plaice (Pleuronectes platessa) | 13–14 (NBE) | Nissling et al. (2002) |
Fig. 5.
Length-at-age (cm, mean with 95% confidence interval limits) of Baltic herring in 1984, 1990, 1997, and 2010 and mean fish weight (g) in the spawning population during 1984–2012. Fish were collected from the spawning grounds in the Archipelago Sea in Southwest Finland (updated on the basis of Rajasilta et al., 1999).
3. Projected changes
3.1. Surface salinity models
We assume that these documented phenomena anticipate future ecosystem changes, and that expectable S changes could be modelled based on projected climate changes. Thus, SSS changes are modelled, based on four climate change scenarios between 1960 and 2100. Four transient simulations for 1960–2100 are performed following Meier et al. (2012) to model changes of the SSS (0–3 m depth). Regionalized data from two global climate models, HadCM3 (Gordon et al., 2000) and ECHAM5/MPI-OM (Roeckner et al., 2006), assuming either the A1B or A2 greenhouse gas emission scenarios (Nakićenović et al., 2000), are used as atmospheric forcing (Meier et al., 2011) for the three- dimensional ocean circulation model RCO, the Rossby Centre Ocean model (Meier et al., 2003). RCO is a Bryan–Cox–Semtner primitive equation circulation model with a free surface and open boundary conditions in the northern Kattegat (Webb et al., 1997). In this study, RCO is used with a horizontal resolution of 3.7 km (2 nautical miles) and with 83 vertical levels with thicknesses of 3 m. For a thorough evaluation of simulated salinity during 1961–2007 the reader is referred to Väli et al. (2013). For further details of the applied dynamical downscaling approach, the reader is referred to Meier et al., 2006, Meier et al., 2012. Runoff changes are calculated with a large-scale hydrological model for the Baltic catchment area (Lindström et al., 2010).
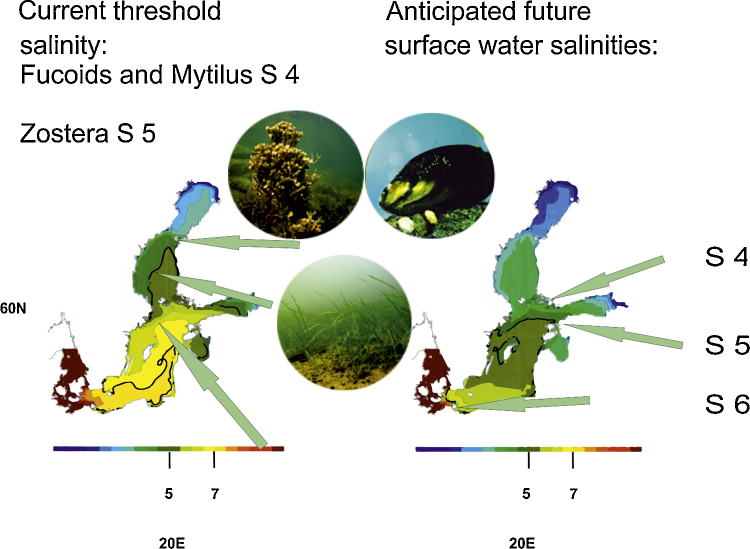
For this study, four scenarios with projected runoff increases, between four and 13 percent, are used in order to model future changes of the surface water S. Projected SSS for 2070–2100 indicate a profound change in the extent of the horohalinicum (Fig. 6). Three out of four of these scenarios project S of less than seven for the entire Baltic Sea surface water around the year 2060 (Fig. 6). The projected movement of the horohalinicum, between now and the end of the century, should show the low-S end (SSS of about 5) to shift from the mid Bothnian Sea southwards to north of the Island of Gotland. The high-S end (SSS of about 7) is likewise expected to retreat from its present position southwards, to the eastern parts of the Danish Straits (Fig. 7). If both of the borders shift about 300–500 km in N–S and S–W directions, this would mean a large change in the horohalinicum. While the overall area of horohalinicum will not necessarily increase, the effect on plant and animal species composition should be remarkable because the area where S is less than seven is expected to expand, considerably. Currently an area of about 65 percent of the Baltic Sea (including the Gulf of Bothnia) surface water has SSS of less than seven (Fig. 2), but virtually the whole Baltic Sea surface layer (Fig. 6, Fig. 7) is, according to our models, projected to have that S, only after some decades with proceeding climate change.
Fig. 6.
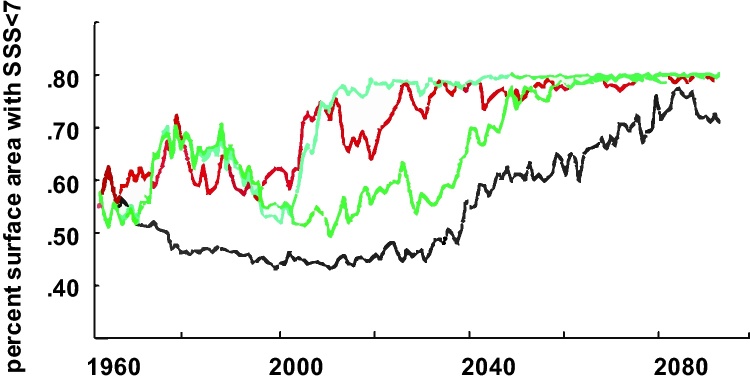
Percent surface area (Y-axis) of the Baltic Sea (including the Kattegat) with salinity of less than seven in four transient scenario simulations from 1960 to 2100 with total runoff increases of four (black line), seven (green), ten (red) and thirteen (turquoise) percent. See Fig. 6 for an indication of the absolute change in area with SSS > 7. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 7.
Ensemble mean SSS calculated from the four projections shown in Fig. 5 and location of the horohalinicum (the area with 7 > SSS > 5) during 1978–2007 (left) and 2070–2099 (right). Several marine foundation species are foreseen to shift southwards by hundreds of kilometres as the isohaline 4 limits Fucus vesiculosus and the blue mussel Mytilus sp., and the isohaline 5 will be limiting the eelgrass Zosteramarina.
3.2. Benthic foundation species and their associates in relation to projected isohalines
We assume effects of salinity to be the same in the future and since we foresee decreasing salinity, we expect species distribution etc. to change accordingly. Several laboratory experiments exist investigating the effects of S on invertebrates (see the Introduction), but the results are not supported by field surveys. On the other hand, numerous field expeditions have produced simple map presentations, where species distributions are also in relation to SSS isohalines (e.g., Segerstråle, 1969, Järvekülg, 1979, Furman et al., 2001, BACC, 2008). The observed S changes considered together with species’ ranges might indicate what may appear under a possible future climate. Recent changes in the biota of the northern Baltic might reappear in future in southern areas, if the S will further decrease. The expected new distribution areas of predominant Baltic Sea animal and plant species can tentatively be placed on a map together with the projected SSS. We collected map-based and other published material regarding the distribution ranges of the predominant species associated with the horohalinicum (Table 1).
Next to the low end of the horohalinicum, several benthic foundation species associate. Foundation species (Dayton, 1972) form, physically, a habitat for other species, thus creating locally favorable conditions for them, for example, in the Baltic Sea the eelgrass, blue mussel, and fucoid algae. Taking the projected S changes as given, the low-S threshold may produce a respective shift in distribution areas of both the foundation species and their associates (Fig. 7).
On sandy bottoms, the eelgrass, Zostera marina, is a foundation species, whose distribution is limited by the S of five (Table 1, Fig. 7). Zostera supports some 30 epifaunal and 20 infaunal species (Boström and Bonsdorff, 1997, Boström et al., 2006, Gustafsson and Boström, 2010). Predominant sand bottom invertebrates, the bivalves Mya arenaria and Cerastoderma spp. and the decapod Crangon grangon, are limited roughly by the same S (Järvekülg, 1979) and probably follow the projected new distribution of Zostera (Table 1). Sandy bottoms are important reproduction and nursery areas for flatfish like turbot (Scophthalmus maximus) and flounder (Pleuronectes flesus), they can be expected to gradually disappear from fish catches in the northern and central Baltic, due to their spawning areas moving south (Nissling et al., 2002, Nissling et al., 2006). The reproductive success of turbot decreases significantly in salinities below seven (Nissling et al., 2006). S restricts also the reproductive success of the flounder, although it can reproduce in somewhat lower S than turbot, by greater than or equal to a S of six (Nissling et al., 2002). Other flatfish species, like dab (Limanda limanda) and plaice (Pleuronectes platessa) require high S for successful reproduction (Table 1), which limits their distribution on south-north axis even in periods when S is higher than now.
Baltic Sea littoral biodiversity is largely associated with the Fucus-zone (Segerstråle, 1928, Segerstråle, 1944, Jansson, 1972 Kangas et al., 1982 Kautsky et al., 1992) with more than 30 associated species. Two Fucus species associated with the horohalinicum (F. radicans and F. vesiculosus) are limited by a minimum S of three and four, respectively (Table 1 and Fig. 7, Bergström et al., 2005, Segerstråle, 1944). Prominent Fucus- associated fauna are the crustaceans (mainly Amphipoda, Isopoda, Mysidacea and Decapoda), of which the gammarids, Gammarus locusta, Leptocheirus pilosus, and Calliopius laeviusculus are limited by salinities of less than five to six (Järvekülg, 1979). The isopods, Idotea baltica and I. viridis, dwell in somewhat lower salinities (Järvekülg, 1979). Finally the Decapod shrimp, Palaemon adspersus is generally limited by minimum salinities of less than five (Järvekülg, 1979). Thus, most of the crustacean biodiversity would follow the retirement of S and the foundation species from the northern Baltic Sea southwards. Fish species, such as perch and pike, forage in the littoral zone among the vegetation and so do the juveniles of many other fish species. The species listed for Amphipoda, Decapoda, and Mysidacea form the basis of littoral fish production (and are crucial for a large coastal fishery in the Baltic Sea). Deeper down on stony and rocky substrates, the predominant species, blue mussel, Mytilus sp. is limited by a S of about four to five (Table 1 and Fig. 7), and provides a habitat for over 40 associated macrofauna species (Westerbom et al., 2002, Koivisto and Westerbom, 2010, Koivisto, 2011).
Some species may virtually disappear east of the Danish Straits, such as the starfish, Asterias rubens, shore crab, Carcinus moenas, and the common periwinkle, Littorina littorea (all limited by S of seven (e.g., Furman et al., 2001). This is expected because their S requirements for reproduction should not be met in the projected future Baltic Sea. This fate may face the hydroid polyps, Laomedea lovéni and L. longissima (limited by salinities of six to seven (Järvekülg, 1979). Hydroid polyps are also a habitat for nudibranch molluscs, Alderia modesta and Limapontia capitata (limiting S is around 5 Seelemann, 1967). They also should retreat from the northern Baltic Sea due to decreasing S.
4. Discussion – possible consequences of the projected salinity changes
Since coastal brackish water areas, globally, are central to the well-being of several aquatic species, important to commercial availability, the issue of an expected increase of freshwater runoff in Northern coastal areas deserves attention. Trying to include a full review of possible implications of our findings for marine ecology, economy, or environmental monitoring would be exhaustive. Instead, we intended to demonstrate some important aspects related to coastal ecology, such as marine fisheries, environmental monitoring, modelling and management, where we conclude that adjustments in current research approaches would improve research performance.
We did not address the concept of horohalinicum (sensu Khlebovich, 1968, or Kinne, 1971), nor the evolutionary explanation of low number of species (Artenminimum) (for a review see, Deaton and Greenberg, 1986), but employed it as a point of focus in order to show some potential effects of S decreases.
Furthermore, we wanted to stress that a projected change in the location of the five to seven practical S units (psu) band of S will not automatically end in a respective shift in all species distributions because of variation among taxa in the possible adaptation and behavioral response to S (e.g., Telesh et al., 2010 Ptacnik et al., 2011 Schubert et al., 2011). Further complications exist in the possible migration of non-indigenous species (Zaiko et al., 2011), adaptations to acid-base changes (Omstedt et al., 2012), eutrophication, and increased temperature (BACC, 2008, Neumann, 2010, Philippart et al., 2011). All these render simple modelling exercises as highly speculative.
The observed S decrease, since the 1970s, cannot be attributed to human-induced climate change, but might instead be caused by internal variations of the climate system. The results of the four scenario simulations (Meier et al., 2012), applied in this study, are within the range of earlier presented S projections of a large multi-model ensemble (Meier et al., 2006). The S decrease may range between present day values and decrease up to 45 percent by the end of the 21st century (BACC, 2008). Thus, we must keep the large uncertainties in the S projections in mind, and that even unchanged S conditions at the end of the 21st century are possible. A more comprehensive discussion about the uncertainties in the modelling is found in Meier et al. (2006).
4.1. Will fresh water species balance the loss of marine fauna?
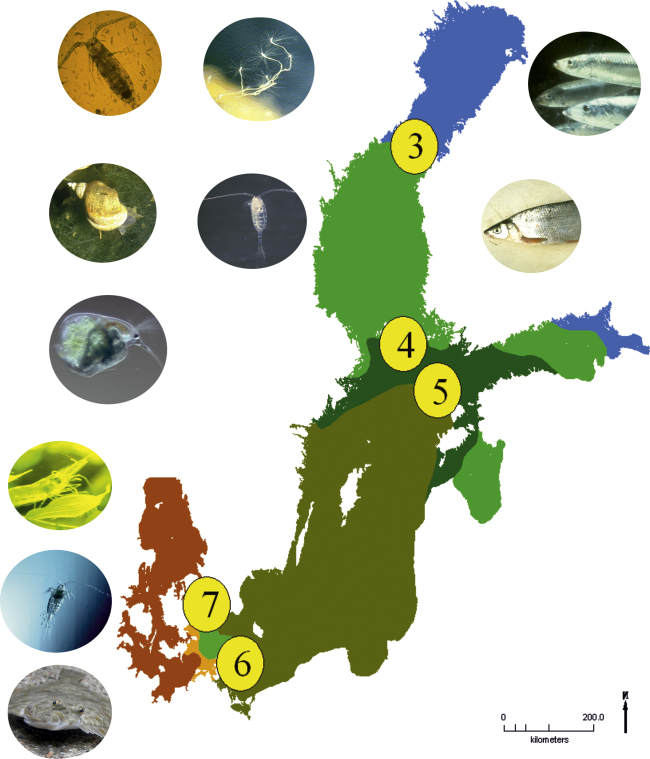
The newly developing Baltic Sea ecosystem, characterized by a reduced S, will encourage invading species from the relatively fresh waters of the Gulf of Finland and Bothnian Bay while several seawater species simultaneously are expected to retreat southwest, and some of them may disappear from the Baltic Sea area. We did not address the possibility of advancing freshwater taxa because relevant biogeographic literature on freshwater fauna and flora on the Baltic Sea coasts is found from national surveys only. Furthermore, the distribution maps of freshwater fauna and flora usually do not take into account the borderline between freshwater and brackish water.The loss of biodiversity, due to retiring marine species, does not necessarily cease biological production, because S will be high enough for several marine species, and furthermore, many freshwater species will be favored, move forward, and replace some of the marine species, disappearing (Fig. 8).
Fig. 8.
Approximate isohalines from 3 to 8 limit the occurrence of typical Baltic Sea plant and animal species around the years 2070–2099. Some species will largely retain their present distribution, such as the Copepod Acartia bifilosa, the Caspian hydroid polyp Cordylophoracaspia and the Baltic herring, as their distribution is limited by isohaline 3 (upper row of inserts). Many freshwater species will advance, such as Gastropods, e.g. Lymnea spp. the copepod Limnocalanus macrurus, and Cladoderans, e.g. Bosmina longispina and cyprinid fish (inserts on the second and third row). Several marine species will experience a migration southwards by hundreds of kilometres. The isohaline 6 is limiting the distribution of flounder. The Decapod shrimps Palemonadspersus and Crangoncrangon will retire around 600 km southwards to isohaline 7, to the level of the Sounds (three inserts lowest in the left corner). Further examples in the text.
For example in the pelagic ecosystem there are eight Calanoid Copepod species in the northern Baltic (Telesh et al., 2008) of which six are of marine origin. The predominant copepods in northern Baltic are Acartia bifilosa and Eurytemora affinis, which both tolerate low salinities (Ackefors, 1969), such as are found today in the Sea of Bothnia. These two are likely to be favored by projected environmental change towards low salinity, as probably will also Cyclopoid copepods, which are predominant in freshwater planktonic environments. On the other hand large calanoid copepods of marine origin, such as Temora longicornis, Centropages hamatus and Pseudocalanus acuspes (Fig. 8) are likely to suffer from low salinities projected for future. While the favorite food item of the Baltic herring, Pseudocalanus acuspes probably will become rare in the Baltic Proper due to limited salinity tolerance of the nauplius larvae (Renz and Hirche 2006), it is likely that Limnocalanus macrurus, and the Cladocera, (esp. Daphnia cucullata and Bosmina longispina) will appear to replace it as staple food for marine pelagic fish. Therefore the production of planktivores e.g. herring does not necessarily crash after the projected change. Of the commercially important marine fish species in the Baltic Sea herring seems to be the only one capable of reproducing within the salinity range of the horohalinicum and even below it (Table 1). In the pelagic areas of the Baltic Sea sprat and herring are dominant planktivores and economically important as well. Of fish the herring is likely still to be present, although smaller in size; sprat or cod, instead, will not be part of the projected alternative food web. Together with a decline of marine copepods in the northern Baltic proper, a decrease is expected of the comb jelly Mertensia ovum (limited by salinity of 5.5, Lehtiniemi et al., 2013), and the moon jellyfish Aurelia aurita (limiting salinity is around 5, Furman et al., 2001).
However, the present day Baltic Sea harbors several phyla and other taxa of high order that have none, or relatively few, fresh water representatives. These taxa include the red and brown algae, and important animal groups such as: Echinodermata, Ctenophora, Polychaeta, Amphipoda, Mysidacea, Decapoda, Gastropoda, and Lamellibranchiata. As only very few, or no alternatives exist that could replace the diminishing or disappearing taxa, the species diversity of Baltic Sea marine fauna may decrease in a comprehensive way. Among Fucus-associated fauna, the gastropod Mollusca, such as Theodoxus fluviatilis, Lymnaea spp., and hydrobiid snails are not limited by low salinities (Järvekülg, 1979). Thus, one expectable future change, above the species-level, may be a molluscan expansion happening together with the decreasing variety of crustaceans among Fucus-belts. Another, and possibly more important expansion, may happen with vascular plants and insects, of which no review, or other compiled source of information over the Baltic Sea is known. This deficit of information concerns also the Oligochaeta, a macrobenthic group, rich in freshwater species. More studies on freshwater species are needed to explain this question, but currently the foreseen changes in S give reason to expect a functional change in ecosystems food-webs due to changes in species numbers.
4.2. Implications for fisheries, monitoring, and modelling
In the Baltic Sea freshening of the water has caused both qualitative and quantitative changes in fish fauna. For instance, reduction of growth has decimated approximately 50 percent of the biomass of the herring spawning stock, as demonstrated; e.g., by our herring data from the northern Baltic Sea (Fig. 5). This must affect the energy flows and ecological interactions among the species in the ecosystem, not to mention the consequences for the fisheries and fish processing industry. By now, the commercial fish catch in the Baltic Sea is about 600–1,000,000 tons annually, and perhaps this is an underestimation (Zeller et al., 2011). The majority of the catch consists of marine species: herring, sprat, cod, and flatfishes. Of these, herring will most likely retain its distribution and dominant position in the pelagic ecosystem even in the northern sea areas (Fig. 8), but its body size remains constantly small, keeping also the stock biomass low. The other marine fish species will gradually disappear from the catches with the extension of the horohalinicum from north to south. For the fishing industry the consequences of this development are problematic. The small species pool of the Baltic Sea does not provide many targets for commercial fisheries even in the present conditions, and thus the loss of one species cannot be compensated just by shifting the fishery to another one. The changing distributions of species also increase the distance to the fishing grounds, which requires new investments on fishing gear and vessels. In the northern sea areas, the future development thus calls for administrative and financial measures at national (Baltic countries) and international (European Union) level, to maintain the fisheries sector profitable. Marine biological monitoring programs are designed to follow changes over time in abundance of plankton, benthic animals, and fish, with limited economical resources. Several complications in the straight–forward use of environmental indicators in the monitoring exist. The observed changes in fish stocks, for example, may be due to other factors than environmental change, such as fishing. Nor is the use of benthic animals without special problems, because; e.g., anoxia in bottom waters may annihilate the whole community in short time, after which it is unreasonable to carry on with monitoring benthic animals at that specific site. The case, we presented here, when changes in biota are due to S changes, is a complex problem because environmental monitoring programs are not designed to indicate a possibility that the species composition would change due to other reasons than the parameter monitored; e.g., pollutants or eutrophication. In this case, changing S affects species distribution and abundance favorably or contrary, according to the origin of species, which is either from freshwater or marine sources. Many Baltic Sea species are not monitored at all and this should be corrected. If incorrect species are selected into monitoring programs, they might not react to changing environmental parameters in the expected way, and an ecosystem change may remain undetected. The monitoring programs are designed to demonstrate changes due to environmental threats, most often mentioned, in the Baltic Sea are eutrophication and persistent pollutants, but minimal information pertaining to the sensitivity of monitored taxa in relation to S changes exist. The fact that a monitored species may vary in abundance for several reasons render this a difficult procedure. Speculatively, a combination of physical, chemical, and biological environmental changes may turn any one of above mentioned species into a key species that profoundly affects the ecology of its habitat. We need a heightened, sophisticated approach to monitoring than exists currently. For example, the ecosystem approach of the European Union’s Marine Strategy Framework Directive, MSFD (2008) could be a basis for development. The MSFD is intended to promote sustainable use of the seas and conserve marine ecosystems. The main objective is to reach and maintain Good Environmental Status (GES) in Europe’s seas. GES is described by e.g. biological diversity. We emphasize that with advancing climate change, S changes are likely to affect biodiversity of brackish water environments. This should be accounted for in future monitoring program designs, and these program designs need to be adapted accordingly.
A special case in monitoring the environment is the concept of biodiversity. Biodiversity is one of the descriptors for a balanced environmental status, for instance in the MSFD (2008). The littoral shallow water benthic environment, especially the littoral zone, harbors great biodiversity. Furthermore, all biological production (and biodiversity) is ultimately based on primary production, which takes place in the illuminated surface layer only. We also emphasize that the decrease of foundation species, and a subsequent decrease of biodiversity, both due to S decrease, does not imply a parallel decrease of the quality of the environment (sensu MSFD and other monitoring programmes for biodiversity). Therefore, a direct monitoring of foundation species seems a meaningful step in implementing MSFD in the Baltic Sea and other monitoring programs in comparable, brackish water ecosystems. Covering many more species than is included presently in the monitoring programs would not be feasible economically; therefore our study strongly encourages the inclusion of the foundation species: Zostera, Fucus, and Mytilus in the monitoring time series studies, but also underscores the importance of basic biological research of S tolerance, food-webs, and cascading effects, therein. Considering future modeling studies, the biological ecosystem models applied in coastal seas should include the change of species composition, during a gradual transition in the ecosystem, from marine species to freshwater ones. The diversity of taxa higher than the species level is a special question, as there are phyla that, in the northern Baltic Sea are represented by few or only one species (e.g., Mertensia ovum for Ctenophora and Halicryptus spinulosus for Priapulida). For these taxa, the loss of only one species also means that characteristics of the recent Baltic Sea marine ecosystem are severely reduced, and that the northern Baltic Sea ecosystem, in the future, may be rather characterized as a river mouth than a coastal sea. This aspect of reduced marine biodiversity, due to other factors than strictly degrading ecological quality of the environment, does not exist in monitoring programs. Other brackish water environments, such as lagoons, estuaries, fjords, and coastal river mouths and plumes may exhibit similar changes with proceeding climate changes thus rendering it a global problem.
Acknowledgements
We thank Olavi Sahlsten for fish samples, Leena Laurila, Juha Kääriä and Jukka Nurminen for maps and photographs and Christoffer Boström for references to Zostera. The research presented is part of the project AMBER (Assessment and Modelling Baltic Ecosystem Response) and has received funding from the European Community’s Seventh Framework Programme (FP/2007-2013) under grant agreement no. 217246 made with BONUS, the joint Baltic Sea research and development programme, and from the Swedish Environmental Protection Agency (SEPA, ref. no. 08/390). Robert M. Badeau, Ph.D., of Aura Professional English Consulting, Ltd. is acknowledged for the language content editing of this manuscript.
References
- Ackefors H. Ecological zooplankton investigations in the Baltic proper 1963–1965. Inst. Mar. Res. Lysekil, Ser. Biol. Rep. 1969;18:139. [Google Scholar]
- BACC . Regional Climate Studies. Springer; Berlin: 2008. Assessment of climate change in the Baltic Sea Basin; p. 473. [Google Scholar]
- Bergström L., Tatarenkov A., Johannesson K., Jönsson R.B., Kautsky L. Genetic and morphological identification of Fucus radicans Sp. nov. (Fucales, Phaeophyceae) in the brackish Baltic Sea. J. Phycol. 2005;41:1025–1038. [Google Scholar]
- Boström C., Bonsdorff E. Community structure and spatial variation of benthic invertebrates associated with Zostera marina (L.) beds in the northern Baltic Sea. J. Sea Res. 1997;37:153–166. [Google Scholar]
- Boström C., O’Brien K., Roos C., Ekebom J. Environmental variables explaining structural and functional diversity of seagrass macrofauna in an archipelago landscape. J. Exp. Mar. Biol. Ecol. 2006;335:52–73. [Google Scholar]
- Casini M., Cardinale M., Hjelm J. Inter-annual variation in herring, Clupea harengus, and sprat, Sprattus sprattus, condition in the central Baltic Sea: what gives the tune? Oikos. 2006;112:638–650. [Google Scholar]
- Casini M., Bartolino V., Molinero J.C., Kornilovs G. Linking fisheries, trophic interactions and climate: threshold dynamics drive herring Clupea harengus growth in the central Baltic Sea. Mar. Ecol. Prog. Ser. 2010;413:241–252. [Google Scholar]
- Dayton P.K. Proceedings of the Colloquium on Conservation Problems. Allen Press; Lawrence, Kansas: 1972. Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antarctica; pp. 81–96. [Google Scholar]
- Deaton L.C., Greenberg M.V. There is no horohalinicum. Estuaries. 1986;9:20–30. [Google Scholar]
- Furman, E., Salemaa, H., Välipakka, P., Munsterhjelm, R., 2001. Östersjön. Ekologi och miljö. 22 pages and 23 transparencies. Helsinki. Walter och Andrée de Nottbecks stiftelse. (ISBN: 951–98521–3–1).
- Gordon C., Cooper C., Senior C.A., Banks H., Gregory J.M., et al. The simulation of SST, sea ice extent and ocean heat transports in a version of the Hadley Centre coupled model without flux adjustments. Clim. Dyn. 2000;16:147–166. [Google Scholar]
- Griffin F.J., Pillai M.C., Vines C.A., Kääriä J., Hibbard-Robins T., et al. Effects of salinity on sperm motility, fertilization and development in the Pacific herring, Clupea pallasi. Biol. Bull. 1998;194:25–35. doi: 10.2307/1542510. [DOI] [PubMed] [Google Scholar]
- Gustafsson C., Boström C. Biodiversity influences ecosystem functioning in aquatic angiosperm communities. Oikos. 2010;120:1037–1046. [Google Scholar]
- Hänninen J., Vuorinen I., Hjelt P. Climatic factors in the Atlantic control the oceanographic and ecological changes in the Baltic Sea. Limnol. Oceanogr. 2000;45:703–710. [Google Scholar]
- Hänninen, J., Vuorinen, I., 2011. Time-Varying Parameter Analysis of the Baltic Sea Freshwater Runoffs. Environmental Modelling and Assessment 16 53–60. 10.1007/s10666–010- 9231–5.
- Jansson B.-O. Ecosystem approach to the Baltic problem. Bull. Ecol. Res. Comm. NFR. 1972;16:1–82. [Google Scholar]
- Järvekülg A. Valgus; Tallinn: 1979. Donnaia Fauna Vostochnoǐj Chasti Baltiǐskogo Morya; p. 382. (In Russian) [Google Scholar]
- Kalnay E., Kanamitsu M., Kistler R., Collins W., Deaven D., et al. The NCEP/NCAR 40-year reanalysis project. Bull. Am. Meteorol. Soc. 1996;77:437–471. [Google Scholar]
- Kangas P., Autio H., Hällfors G., Luther H., Niemi Å, et al. A general model of the decline of Fucus vesiculosus at Tvärminne, south coast of Finland, in 1977–1981. Acta Bot. Fenn. 1988;118:1–27. [Google Scholar]
- Kautsky H., Kautsky L., Kautsky N., Kautsky U., Lindblad C. Studies on the Fucus vesiculosus community in the Baltic Sea. Acta Phytogeogr. Suec. 1992;78:33–48. [Google Scholar]
- Karaseva E.M., Ivanovich V.M. Vertical distribution of eggs and larvae of the Baltic sprat Sprattus sprattus balticus (Clupeidae) in relation to seasonal and diurnal variation. J. Ichthyol. 2010;50:259–269. [Google Scholar]
- Khlebovich V.V. Some peculiar features of the hydrochemical regime and the fauna of mesohaline waters. Mar. Biol. 1968;2:47–49. [Google Scholar]
- Kinne O. The effects of temperature and salinity on marine and brackish water animals. II. Salinity and temperature-salinity relations. Oceanogr. Mar. Biol. An Ann. Rev. 1964;2:281–339. [Google Scholar]
- Kinne O., editor. A Comprehensive, Integrated Treatise on Life in Oceans and Coastal waters I. Environmental Factors Part 2:683–1244. Interscience/Wiley-Interscience; London: 1971. Marine ecology. (ISBN: 0–471-48002–9) [Google Scholar]
- Klinkhardt M. Zum einfluss des salzgehaltes auf die befruchtungsfähigkeit des laiches der rügenschen frühjahrsheringe fischerei-forschung. Wissenschaftliche Schriftenreihe. 1984;22:73–75. [Google Scholar]
- Koivisto M.E. Academic Dissertation. Faculty of Biological and Environmental Sciences of the University of Helsinki; 2011. Blue mussel beds as biodiversity hot spots on the rocky shores of the northern Baltic Sea; p. 498. [Google Scholar]
- Koivisto M., Westerbom M. Habitat structure and complexity as determinants of biodiversity in blue mussel beds on sublittoral rocky shores. Mar. Biol. 2010;157:1463–1474. [Google Scholar]
- Lehtiniemi M., Gorokhova E., Bolte S., Haslob H., Huwer B., Katajisto T., Lennuk L., Markkula S., Põllumäe A., Schaber M., Setälä O., Reusch T.B.H., Viitasalo-Frösén S., Vuorinen I., Välipakka P. Distribution and reproduction of the Arctic ctenophore Mertensia ovum in the Baltic Sea ? a species long misidentified as Pleurobrachia pileus. Marine Ecology Progress Series. 2013;491:111–124. [Google Scholar]
- Leppäkoski E., Helminen H., Hänninen J., Tallqvist M. Aquatic biodiversity under anthropogenic stress: an insight from the Archipelago Sea (SW Finland) Biodivers. Conserv. 1999;8:55–70. [Google Scholar]
- Lindström G., Pers C., Rosberg J., Strömqvist J., Arheimer B. Development and testing of the HYPE (hydrological predictions for the environment) water quality model for different spatial scales. Hydrol. Res. 2010;41:295–319. [Google Scholar]
- Meier H.E.M., Döscher R., Faxén T. A multiprocessor coupled ice-ocean model for the Baltic Sea: application to salt inflow. J. Geophys. Res. 2003;108(C8):3273. doi: 10.1029/2000JC000,521. [DOI] [Google Scholar]
- Meier H.E.M., Kjellström E., Graham L.P. Estimating uncertainties of projected Baltic Sea salinity in the late 21st century. Geophys. Res. Lett. 2006;33 doi: 10.1029/2006GL026488. [DOI] [Google Scholar]
- Meier H.E.M., Höglund A., Döscher R., Andersson Y.H., Löptien U., et al. Quality assessment of atmospheric surface fields over the Baltic Sea from an ensemble of regional climate model simulations with respect to ocean dynamics. Oceanologia. 2011;53(1-TI):193–227. [Google Scholar]
- Meier H.E.M., Hordoir R., Andersson H.C., Dieterich C., Eilola K., Gustafsson B.G., Höglund A., Schimanke S. Modeling the combined impact of changing climate and changing nutrient loads on the Baltic Sea environment in an ensemble of transient simulations for 1961–2099. Clim. Dyn. 2012;39:2421–2441. doi: 10.1007/s00382-012-1339-7. [DOI] [Google Scholar]
- Möllmann C., Kornilovs G., Fetter M., Köster F.W. Climate, zooplankton, and pelagic fish growth in the central Baltic Sea. ICES J Mar Sci. 2005;62:1270–1280. [Google Scholar]
- MSFD, 2008. DIRECTIVE 2008/56/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 17 June 2008 establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive).
- Nakićenović N., Alcamo J., Davis G., de Vries B., Fenhann J., et al. A Special Report of Working Group III of the Intergovernmental Panel on Climate Change. Cambridge University Press; 2000. Emission scenarios; p. 599. [Google Scholar]
- Neumann T. Climate-change effects on the Baltic Sea ecosystem: a model study. J. Mar. Sys. 2010;81:213–244. [Google Scholar]
- Nissling A., Johansson U., Jacobsson M. Effects of salinity and temperature conditions on the reproductive success of turbot (Scophthalmus maximus) in the Baltic Sea. Fish. Res. 2006;80:230–238. [Google Scholar]
- Nissling A., Müller A., Hinrichsen H.-H. Specific gravity and vertical distribution of sprat (Sprattus sprattus) eggs in the Baltic Sea. J. Fish. Biol. 2003;63:280–299. [Google Scholar]
- Nissling A., Westin L. Salinity requirements for successful spawning of Baltic and Belt Sea cod and the potential for cod stock interactions in the Baltic Sea. Mar. Ecol. Prog. Ser. 1997;152:261–271. [Google Scholar]
- Nissling A., Westin L., Hjerne O. Reproductive success in relation to salinity for three flatfish species, dab (Limanda limanda), plaice (Pleuronectes platessa), and flounder (Pleuronectes flesus) in the brackish water Baltic Sea. ICES J. Mar. Sci. 2002;59:93–108. [Google Scholar]
- Ojaveer E. Influence of temperature, salinity, and reproductive mixing of Baltic herring groups on its embryonal development. Rapports et Procès-Verbaux des Réunions du Conseil Int. Pour l’Exploration de la Mer. 1981;178:409–415. [Google Scholar]
- Ojaveer H., Jaanus A., MacKenzie B.R., Martin G., Olenin S., et al. Status of biodiversity in the Baltic Sea. PloS ONE. 2010;5(e):12467. doi: 10.1371/journal.pone.0012467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojaveer H., Lumberg A., Ojaveer E. Highlights of zooplankton dynamics in Estonian waters (Baltic Sea) ICES J. Mar. Sci. 1998;55:748–755. [Google Scholar]
- Omstedt A., Edman M., Claremar B., Frodin P., Gustafsson N.E., Humborg C., Hägg H., Mörth M., Rutgersson A., Schurgers G., Smith B., Wällstedt T., Yurova A. Future changes in the Baltic acid-base (pH) and oxygen balances. Tellus B. 2012;64(2012):19586. doi: 10.3402/tellusb.v64io.19586. [DOI] [Google Scholar]
- Ptacnik R., Olli K., Lehtinen S., Tamminen T., Andersen T. Does plankton diversity peak at intermediate salinities? Comment on Telesh et al. (2011) Mar. Ecol. Prog. Ser. 2011;432:291–292. [Google Scholar]
- Philippart C.J.M., Anadón R., Danovaro R., Dippner J.W., Drinkwater K.F., Hawkins S.J., Oguz T., O’sullivan G., Reid P.C. Impacts of climate change on European marine ecosystems: observations expectations and indicators. J. Exp. Mar. Biol. Ecol. 2011;400:552–569. [Google Scholar]
- Rajasilta M., Jönsson N., Eklund J., Lorenz T., Laine P. Study report EU 96–068. Archipelago Research Institute, University of Turku; 1999. Intensive monitoring of spawning populations of the Baltic herring (Clupea harengus membras L.) [Google Scholar]
- Renz J., Hirche H.J. Life cycle of Pseudocalanus acuspes Giesbrecht (Copepoda, Calanoida) in the Central Baltic Sea: I. Seasonal and spatial distribution. Mar. Biol. 2006;148:467–580. [Google Scholar]
- Remane, A., Schlieper, C., 1971. Biology of Brackish Water. (2nd revised ed.) Die Binnengewässer XXV. 372 pp. Schweizerbart’sce Verlagsbuchandlung (Nägele u. Obermiller) Stuttgart.
- Roeckner E., Brokopf R., Esch M., Giorgetta M., Hagemann S., et al. Sensitivity of simulated climate to horizontal and vertical resolution in the ECHAM5 atmosphere model. J. Climatol. 2006;19:3771–3791. [Google Scholar]
- Schubert H., Feuerpfeil P., Marquardt R., Telesh I., Skarlato S. Macroalgal diversity along the Baltic Sea salinity gradient challenges Remane’s species-minimum concept. Mar. Pollut. Bull. 2011;62(9):1948–1956. doi: 10.1016/j.marpolbul.2011.06.033. [DOI] [PubMed] [Google Scholar]
- Seelemann U. Rearing experiments on the amphibian slug Alderia modesta. Helgol. Mar. Res. 1967;15:128–136. [Google Scholar]
- Segerstråle, S.G., 1928. Quantitative Studien űber den Tierbestand der Fucus-Vegetation in den Schären von Pellinge (an der Sűdkűste Finnlands). Societas Scientiarum Fennica Commentationes biologicae 3:2.
- Segerstråle, S.G., 1944. Weitere Studien űber die Tierwelt der Fucus- Vegetation an der Sűdkűste Finnlands. Societas Scientiarum Fennica Commentationes Biologicae 9:4.
- Segerstråle S.G. Baltic Sea. Geolog. Soc. Am. Mem. 1957;67:751–800. [Google Scholar]
- Segerstråle S.G. Biological fluctuations in the Baltic Sea. Prog. Oceanogr. 1969;5:169–184. [Google Scholar]
- Telesh I., Postel L., Heerkloss R., Mironova E., Skarlato S. Zooplankton of the Open Baltic Sea: Atlas. BMB Publication No. 20 Meereswiss. Ber. Warnemünde. 2008;73:1–251. [Google Scholar]
- Telesh I.V., Schubert H., Skarlato S.O. Revisiting Remane’s concept: evidence for high plankton diversity and a protistan species maximum in the horohalinicum of the Baltic Sea. Mar. Ecol. Prog. Ser. 2010;421:1–11. [Google Scholar]
- Vuorinen I., Hänninen J., Viitasalo M., Helminen U., Kuosa H. Proportion of copepod biomass declines with decreasing salinity in the Baltic Sea. ICES J. Mar. Sci. 1998;55(4):767–777. [Google Scholar]
- Väli G., Meier H.E.M., Elken J. Simulated halocline variability in the Baltic Sea and its impact on hypoxia during 1961–2007. J. Geophys. Res. 2013;118:6982–7000. doi: 10.1002/2013JC009192. [DOI] [Google Scholar]
- Webb D.J., Coward A.C., de Cuevas B.E., Gwilliam C.S. A multiprocessor ocean circulation model using message passing. J. Atmos. Oceanic Technol. 1997;14:175–183. [Google Scholar]
- Westerbom M., Kilpi M., Mustonen O. Blue mussels, Mytilus edulis, at the edge of the range: population structure growth and biomass along a salinity gradient in the north-eastern Baltic Sea. Mar. Biol. 2002;140:991–999. [Google Scholar]
- Zaiko A., Lehtiniemi M., Narščius A., Olenin S. Assessment of bioinvasion impacts on a regional scale: a comparative approach. Biol. Inv. 2011 doi: 10.1007/s10530-010-9928-z. [DOI] [Google Scholar]
- Zeller D., Rossing P., Harper S., Persson L., Booth S., Pauly D. The Baltic Sea: estimates of total fisheries removals 1950–2007. Fish. Res. 2011;108:356–363. [Google Scholar]