Abstract
Importance
Anti-N-methyl-d-aspartate receptor (anti-NMDAR) autoimmune encephalitis is an increasingly recognized cause of limbic encephalitis (LE). Prolonged LE and limbic status epilepticus (LSE) share many features. The ability to distinguish between the two is crucial in directing appropriate therapy because of the potential iatrogenesis associated with immunosuppression and anesthetic-induced coma.
Observations
A 34-year-old woman with recurrent LE developed behavioral changes, global aphasia, and repetitive focal and generalized tonic–clonic seizures. Because asymmetric rhythmic delta patterns recurred on electroencephalography (EEG) despite treatment with nonsedating antiepileptic drugs followed by anesthetic-induced coma, an investigation to distinguish LSE from LE was undertaken. Implanted limbic/temporal lobe depth electrodes revealed no epileptiform activity. Brain single-photon emission computerized tomography (SPECT) showed no hyperperfusion, and brain fluorodeoxyglucose-positron emission tomography (FDG-PET) showed hypermetabolism in the left frontal, temporal, and parietal cortices. Anti-N-methyl-d-aspartate receptor autoimmune encephalitis was diagnosed based detection of anti-NMDAR antibody in the cerebrospinal fluid (CSF). With chronic immunosuppression, the resolution of brain FDG-PET abnormalities paralleled clinical improvement.
Conclusions and relevance
This case of anti-NMDAR autoimmune encephalitis illustrates the challenges of distinguishing prolonged LE from LSE. We discuss the parallels between these two conditions and propose a management paradigm to optimize evaluation and treatment.
Keywords: Anti-NMDA receptor antibodies, Autoimmune encephalitis, Limbic encephalitis, Limbic status epilepticus
1. Introduction
Limbic encephalitis (LE) is considered in patients with short-term memory loss, confusion, behavioral changes with irritability, depression, sleep disturbance, hallucinations, orofacial dyskinesias, and seizures involving the medial temporal lobes and amygdala [1], [2]. Encephalitis with anti-N-methyl-d-aspartate receptor (anti-NMDAR) antibodies is an autoimmune, and often paraneoplastic, form of LE with prodromal viral-like illness, prominent impaired consciousness, abnormal movements, and autonomic instability [3]. Prolonged states of behavioral changes, orofacial movements, and partial seizures in LE suggest underlying limbic status epilepticus (LSE) [4]. When facing the diagnostic dilemma of autoimmune LE and LSE, antiepileptic and immunosuppressive treatment is often initiated while awaiting confirmatory antibody titers. However, patients may develop refractory or malignant features with diagnostically ambiguous rhythmic activity on electroencephalography (EEG). The clinician must weigh the morbidity of ongoing limbic seizures against the morbidity of anesthetic-induced coma in an effort to overcome suspected LSE. Here, we present a case where this conundrum was explored using surface and depth electrode EEG. We propose a paradigm for distinguishing LE from LSE.
2. Report of a case
A 34-year-old woman with a history of two episodes of LE in the prior nine years developed gradual onset difficulty with concentration, mild headaches, and hemibody paresthesias that progressed to include orofacial dyskinesias, hemiparesis, global aphasia, and repetitive focal and generalized tonic–clonic seizures. Her previous episodes of LE, from which she made a complete recovery, were characterized by behavioral changes, aphasia, and focal and generalized tonic–clonic seizures. She had a family history of autoimmunity, with a mother with Sjögren's disease and a maternal aunt with systemic lupus erythematosus. Her initial evaluation revealed a cerebrospinal fluid (CSF) lymphocytic pleocytosis (46 leukocytes/mm3) and elevated protein (95 mg/dL), with negative anti-NMDAR serologies. Fluorodeoxyglucose-positron emission tomography (FDG-PET) performed during the first clinical episode demonstrated multiple FDG-avid hypermetabolic areas involving the right frontal, temporal, and parietal lobes. She was treated with high-dose intravenous (IV) corticosteroids, intravenous immunoglobulin (IVIg), and oral corticosteroid taper. A corticomeningeal biopsy was nondiagnostic. The brain FDG-PET abnormality resolved prior to the second clinical episode. She was subsequently treated with a regimen of lamotrigine, carbamazepine, and oral prednisone.
Five years after her second clinical episode, she experienced trouble concentrating at work with intermittent headaches and presented to her neurologist for evaluation. Outpatient brain magnetic resonance imaging (MRI) revealed a T2 hyperintensity in the left parietal lobe that prompted hospitalization and CSF analysis showing a lymphocytic pleocytosis (103 leukocytes/mm3) and normal protein (42 mg/dL). By report, EEG demonstrated intermittent irregular slowing over the left temporal region, without epileptiform discharges. Following high-dose IV corticosteroids, she was discharged on oral corticosteroid taper, carbamazepine, and lamotrigine. She continued to have language deficits, right arm weakness, and difficulty with feeding and dressing herself. A follow-up outpatient brain MRI was normal. Within weeks, she developed episodic right face twitching, right hemibody paresthesias with hemiparesis, and generalized tonic–clonic seizures. She was readmitted, given IV antiepileptic drugs, and reinitiated on high-dose IV corticosteroids. Per report, repeat CSF studies and EEG were unchanged from prior studies, showing lymphocytic pleocytosis (76 leukocytes/mm3) with normal protein (36 mg/dL) and focal left temporal slowing. Following prolonged right facial twitching and generalized abnormal movements, IVIg was initiated, and she was transferred to our hospital for further evaluation and management.
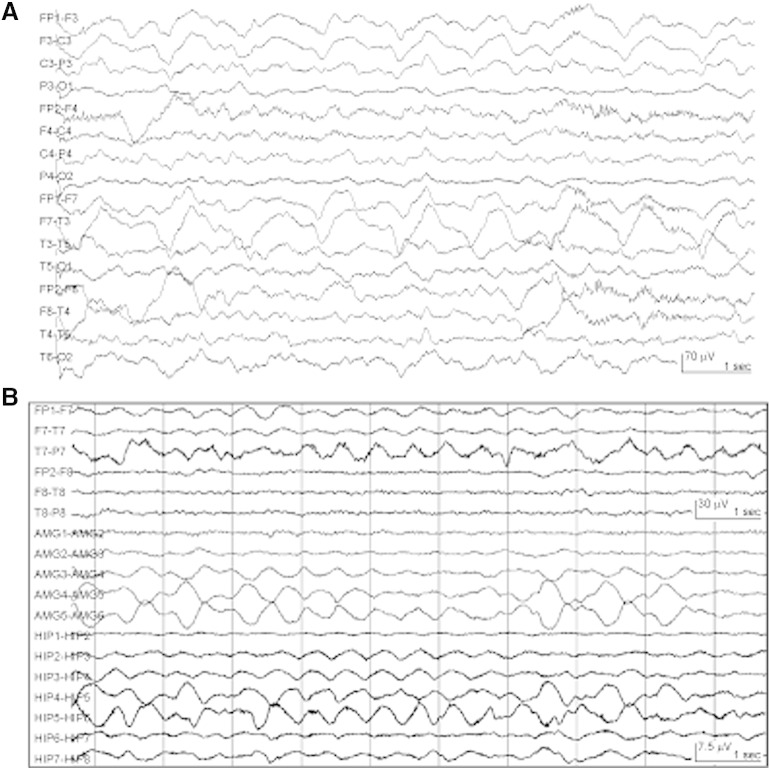
On admission, she was alert and nonverbal with global aphasia. Intermittent right facial twitches and right arm movements were noted. Motor examination showed antigravity strength in the left hemibody and right leg. No purposeful movements of the right arm were noted. Electrographic seizures with clinical correlates were captured on EEG, so her antiepileptic regimen was escalated. On subsequent continuous EEG monitoring, intermittent rhythmic left temporal delta activity was seen (Fig. 1A), without sharps or epileptiform waveforms. Repeat CSF analysis showed persistent lymphocytic pleocytosis (53 leukocytes/mm3), and serologic inflammatory and rheumatologic markers were normal. A brain single-photon emission computerized tomography (SPECT) study showed no abnormal perfusion (data not shown). Clinically, she had fluctuating worsening of her aphasia and right arm weakness that correlated with temporal delta activity that would progress and evolve asymmetrically and rhythmically in bursts of 10 s. Levetiracetam, fosphenytoin, valproic acid, lacosamide, and continuous midazolam infusion failed to suppress this activity. She completed a 2-g/kg course of IVIg and five days of high-dose IV methylprednisolone and was treated with prednisone 60 mg daily. Based on concern for LSE, propofol was used to suppress the rhythmic delta activity, but this recurred with each attempt at propofol weaning and despite addition of phenobarbital. As discussed elsewhere, the ketogenic diet was initiated for super-refractory SE [5]. After a total of eight weeks without clinical or EEG remission and despite multiple rounds of anesthetic-induced electrographic burst suppression, a point of risk/benefit equipoise was raised. The iatrogenesis of further anesthetic-induced coma with immunosuppressant treatment was thought to possibly outweigh the ambiguity of treating rhythmic delta activity at the scalp surface on the assumption that this represented LSE at the depths, particularly in the case of idiopathic LE. Depth electrode monitoring was utilized to determine if the rhythmic delta activity was indeed epileptic since rhythmic delta activity can be a scalp ictal pattern for seizures originating from mesial temporal structures [6].
Fig. 1.
Continuous surface and depth electrode electroencephalographic monitoring in anti-NMDAR autoimmune encephalitis. (A) Scalp EEG monitoring demonstrated delta slowing that was maximal over the left temporal area. (B) Surgically implanted depth electrodes targeting the left amygdala (AMG) and left hippocampus (HIP) showed nearly continuous semirhythmic to rhythmic delta activity that correlated with surface EEG slowing.
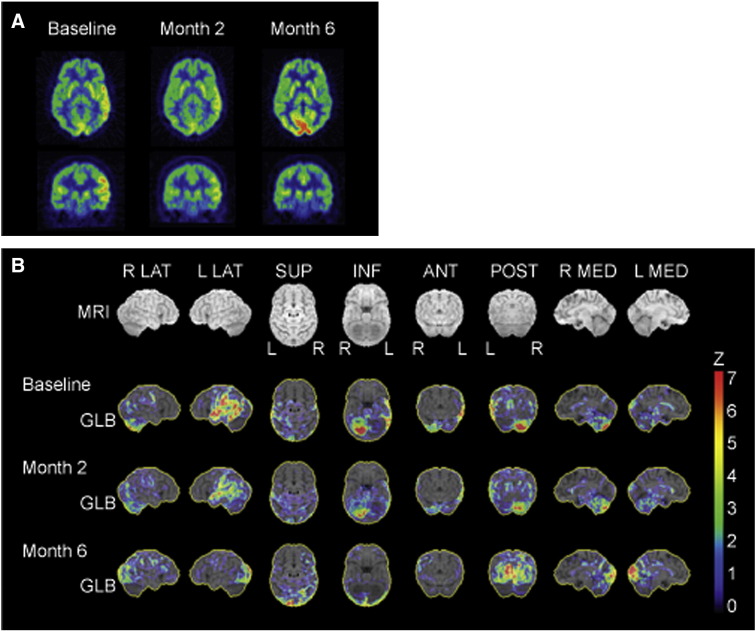
During 14 days of simultaneous continuous monitoring of scalp EEG and surgically implanted intracranial depth electrodes targeting the left amygdala and left hippocampus, no spike or spike–wave activity was recorded. Rather, both surface and depth electrodes demonstrated near continuous semirhythmic to rhythmic slow delta activity that was maximal over the left temporal area on scalp EEG (Fig. 1B). Notably, brain FDG-PET showed asymmetric FDG-avidity in the left frontal, temporal, and parietal cortices (Figs. 2A and B). In the absence of clear ictal patterns, this increased metabolic activity was attributed to LE, and the ketogenic diet and phenobarbital were tapered off. Given concern for an autoimmune etiology, the patient was treated with five exchange courses of plasmapheresis. When the anti-NMDAR antibody test returned positive from her CSF (not serum), she was initiated on rituximab, started on a slow oral prednisone taper, and transitioned to levetiracetam and lamotrigine for seizure prophylaxis. Abdominal and transvaginal ultrasounds and pelvic MRI were unremarkable. Whole-body FDG-PET/CT failed to reveal occult malignancy. Her aphasia gradually improved over six months, along with improvements in the metabolic asymmetry on brain FDG-PET (Figs. 2A and B).
Fig. 2.
Serial brain fluorodeoxyglucose-positron emission tomography image analysis in anti-NMDAR autoimmune encephalitis. (A) Representative transaxial and coronal FDG-PET images obtained at indicated times from symptom onset. Baseline scan showed increased FDG-avid hypermetabolism in the left temporal and parietal regions. With chronic immunosuppression, these hypermetabolic cortical abnormalities essentially resolved by six months. (B) Statistical images were generated by three-dimensional stereotactic surface projection (3D-SSP) analysis using NEUROSTAT (provided by the Department of Radiology, University of Washington, Seattle, WA, USA). Images represent Z scores above the mean compared with an age-matched control database. All images were normalized to global brain activity (GLB). Baseline FDG-PET images demonstrated asymmetric hypermetabolism in the left temporal, parietal, and frontal cortices. Serial FDG-PET imaging at two and six months after symptom onset showed interval resolution of FDG-avid cortical asymmetry. There was also right cerebellar hypermetabolism (consistent with crossed diaschisis) that improved over serial scans.
3. Discussion
We present a patient with recurrent episodes of LE, where no etiology had been identified after multiple hospitalizations over nine years. The ultimate diagnosis of anti-NMDAR autoimmune encephalitis by detection of the autoantibody in CSF confirms prior reports and emphasizes the importance of testing CSF and serum when evaluating patients in whom autoimmune LE is suspected [7]. Notably, her serum remained negative for anti-NMDAR as well as other autoantibodies.
The EEG is typically abnormal in anti-NMDAR encephalitis [7], with predominantly low-voltage delta to theta range activity [8], [9], [10], [11]. Here, we demonstrate that the rhythmic delta activity on surface EEG in a patient with anti-NMDAR autoimmune encephalitis was not associated with evidence of cyclic spike or spike–wave activity on depth recording. This important observation addresses the question as to whether rhythmic slowing on the surface EEG represents LSE or a form of nonconvulsive status epilepticus in anti-NMDAR encephalitis [4], [9].
Brain PET FDG-avid hypermetabolism in mesiotemporal structures has been reported in autoimmune LE and has been correlated with focal slow activity on surface EEG in anti-NMDAR encephalitis [12], [13], [14]. With serial brain FDG-PET scans, we found asymmetric cortical hypermetabolism in anti-NMDAR encephalitis, and the rhythmic slowing in depth and surface EEG was attributed to encephalitic changes. Indeed, it was with escalation of immunotherapy and optimization of prophylactic antiepileptic drugs that our patient began to improve clinically. Furthermore, the resolution of the brain FDG-PET abnormalities paralleled the amelioration of symptoms with chronic suppression immunotherapy.
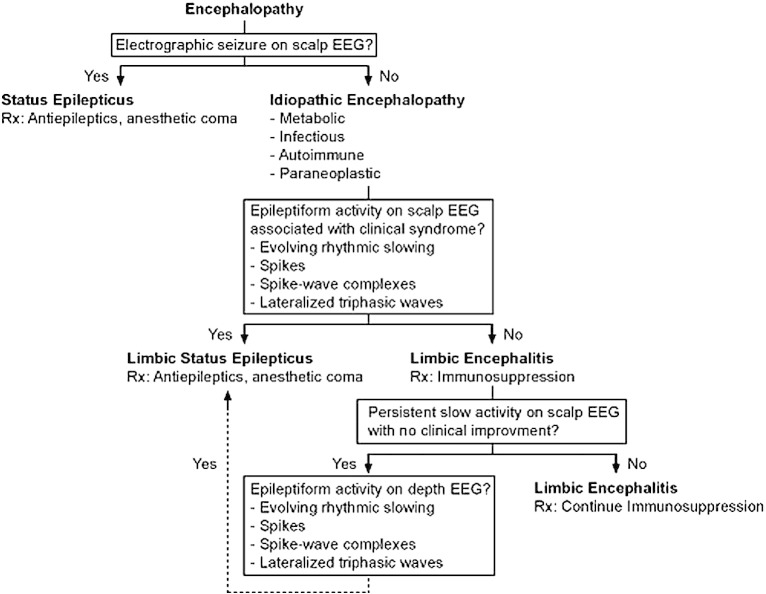
The lessons learned from this case inform a diagnostic and therapeutic approach (Fig. 3) that focuses on efficient and deliberate interventions to reduce potential iatrogenic morbidity and, conversely, to improve appropriate management in cases of suspected autoimmune LE, particularly in anti-NMDAR encephalitis [7], [15]. In prolonged anti-NMDAR LE, when rhythmic delta activity persists despite anesthetic-induced burst suppression and immunotherapy, the use of depth electrodes has been proposed to help distinguish LE from LSE [4]. We present a patient in whom persistent surface rhythmic delta activity did not correlate with depth spike or spike–wave activity but was concurrent with depth recording of rhythmic delta activity. Clinical improvement was realized with the introduction of second-line immunotherapies, allowing for optimization of the antiepileptic regimen. We outline a paradigm that may be helpful in managing autoimmune LE and distinguishing features from LSE.
Fig. 3.
Diagnostic and therapeutic approach to patients with suspected autoimmune limbic encephalitis.
Article information
Study concept and design: Probasco, Benavides, and Kaplan.
Acquisition of data: Probasco, Benavides, Wills, Ciarallo, and Wabulya.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: Probasco, Benavides, and Kaplan.
Critical revision of the manuscript for important intellectual content: All authors.
Study supervision: Bergey and Kaplan.
Disclosures
Drs. Probasco, Benavides, Ciarallo, Wills, and Wabulya have no disclosures to report.
Dr. Bergey receives editorial fees as Associate Editor of the journal Neurotherapeutics and serves as a Neuropace Advisory Board Member but has received no compensation.
Dr. Kaplan reports grants from Qatar National Research Foundation (EEG in the ICU for nonconvulsive status epilepticus); personal fees from Royalties Wiley, Demos for books on epilepsy and EEG; honoraria for international congresses and grand rounds (Europe and North America); travel fee to give lectures (Europe and North America); consulting fee for EEG reading (Eisai Pharma); expert testimony on qEEG in the courtroom; and no financial support for board membership on the ACNS and ABCN.
Conflict of interest statement
The authors report no conflicts of interest in relation to this case report.
References
- 1.Gultekin S.H., Rosenfeld M.R., Voltz R., Eichen J., Posner J.B., Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123(Pt 7):1481–1494. doi: 10.1093/brain/123.7.1481. [DOI] [PubMed] [Google Scholar]
- 2.Graus F., Delattre J.Y., Antoine J.C., Dalmau J., Giometto B., Grisold W. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75(8):1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalmau J., Gleichman A.J., Hughes E.G., Rossi J.E., Peng X., Lai M. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan P.W., Rossetti A.O., Kaplan E.H., Wieser H.G. Proposition: limbic encephalitis may represent limbic status epilepticus. A review of clinical and EEG characteristics. Epilepsy Behav. 2012;24(1):1–6. doi: 10.1016/j.yebeh.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Thakur K.T., Probasco J.C., Hocker S.E., Roehl K., Henry B., Kossoff E.H. Ketogenic diet for adults in super-refractory status epilepticus. Neurology. 2014;82(8):665–670. doi: 10.1212/WNL.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung K.Y., Kang J.K., Kim J.H., Im C.H., Kim K.H., Jung H.K. Spatiotemporospectral characteristics of scalp ictal EEG in mesial temporal lobe epilepsy with hippocampal sclerosis. Brain Res. 2009;1287:206–219. doi: 10.1016/j.brainres.2009.06.071. [DOI] [PubMed] [Google Scholar]
- 7.Titulaer M.J., McCracken L., Gabilondo I., Armangue T., Glaser C., Iizuka T. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kataoka H., Takatani T., Ueno S. Low-voltage EEG activity presenting from psychotic stage in a patient with anti-NMDA receptor encephalitis. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr-2012-007045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkpatrick M.P., Clarke C.D., Sonmezturk H.H., Abou-Khalil B. Rhythmic delta activity represents a form of nonconvulsive status epilepticus in anti-NMDA receptor antibody encephalitis. Epilepsy Behav. 2011;20(2):392–394. doi: 10.1016/j.yebeh.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Johnson N., Henry C., Fessler A.J., Dalmau J. Anti-NMDA receptor encephalitis causing prolonged nonconvulsive status epilepticus. Neurology. 2010;75(16):1480–1482. doi: 10.1212/WNL.0b013e3181f8831a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan P.W., Sutter R. Electroencephalography of autoimmune limbic encephalopathy. J Clin Neurophysiol. 2013;30(5):490–504. doi: 10.1097/WNP.0b013e3182a73d47. [DOI] [PubMed] [Google Scholar]
- 12.Baumgartner A., Rauer S., Mader I., Meyer P.T. Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol. 2013;260(11):2744–2753. doi: 10.1007/s00415-013-7048-2. [DOI] [PubMed] [Google Scholar]
- 13.Mohr B.C., Minoshima S. F-18 fluorodeoxyglucose PET/CT findings in a case of anti-NMDA receptor encephalitis. Clin Nucl Med. 2010;35(6):461–463. doi: 10.1097/RLU.0b013e3181db4d4a. [DOI] [PubMed] [Google Scholar]
- 14.Greiner H., Leach J.L., Lee K.H., Krueger D.A. Anti-NMDA receptor encephalitis presenting with imaging findings and clinical features mimicking Rasmussen syndrome. Seizure. 2011;20(3):266–270. doi: 10.1016/j.seizure.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Irani S.R., Bera K., Waters P., Zuliani L., Maxwell S., Zandi M.S. N-methyl-d-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133(Pt 6):1655–1667. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]