Figure 2. Bioorthogonal reactions occur selectively between two abiotic functional-groups.
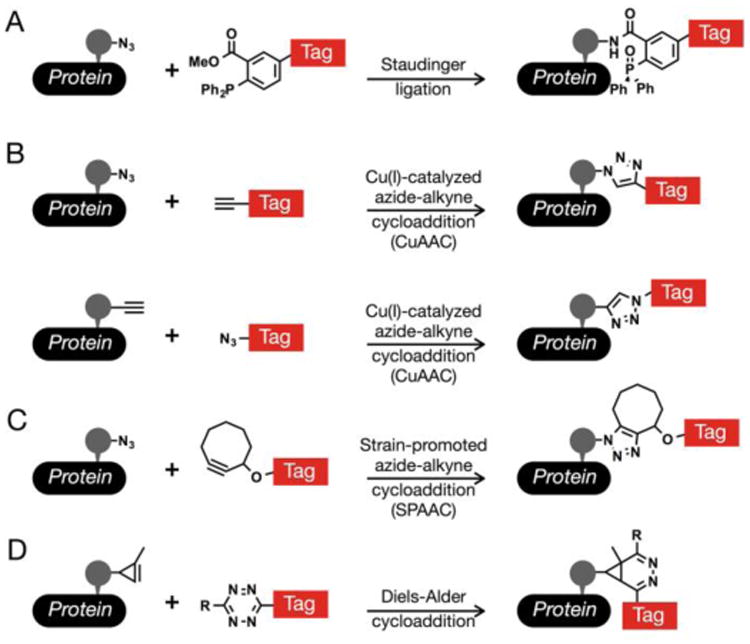
(A) The Staudinger ligation allows for the coupling of azides with triarylphosphine reagents. (B) The Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) gives a stable triazole product from azides and alkynes. This common bioorthogonal reaction can be performed in both directions; however, azide-tags give reduced background signals. (C) Cyclooctyne reagents will react with azides in a strain-promoted azide-alkyne cycloaddition (SPAAC). (D) Tetrazines will undergo rapid inverse-demand Diels-Alder reactions with activated alkyenes, such as cyclopropenes.
