Abstract
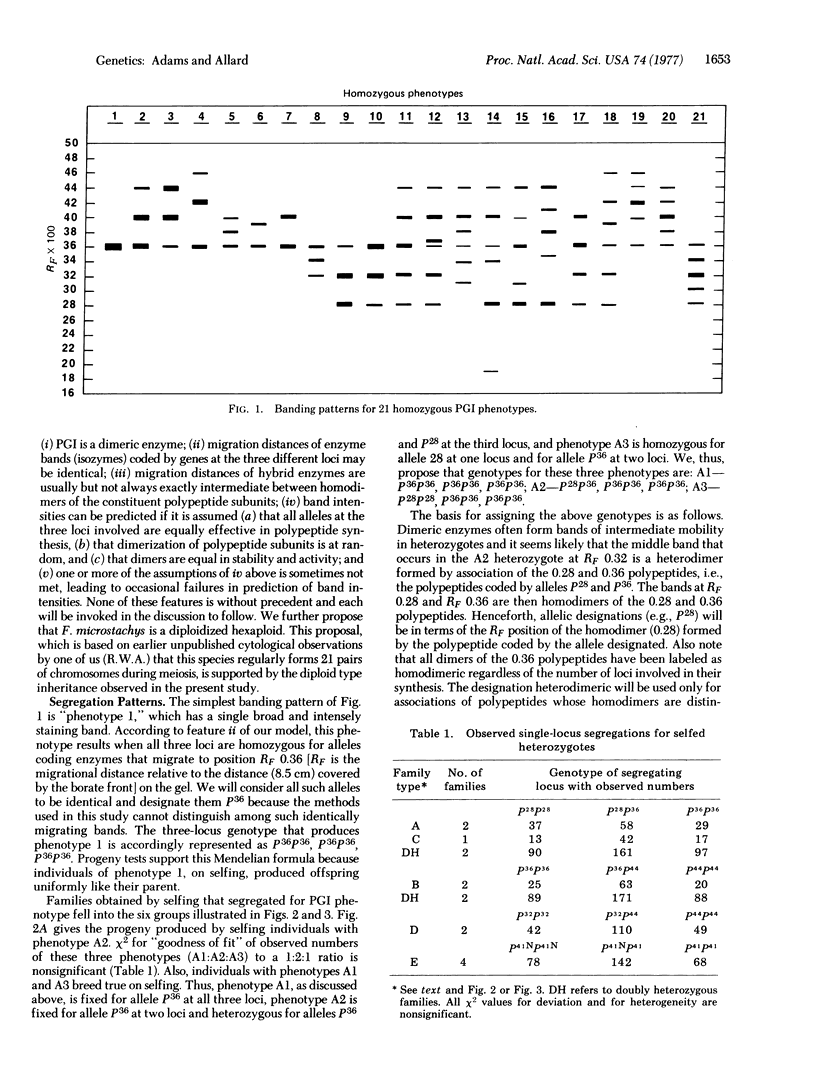
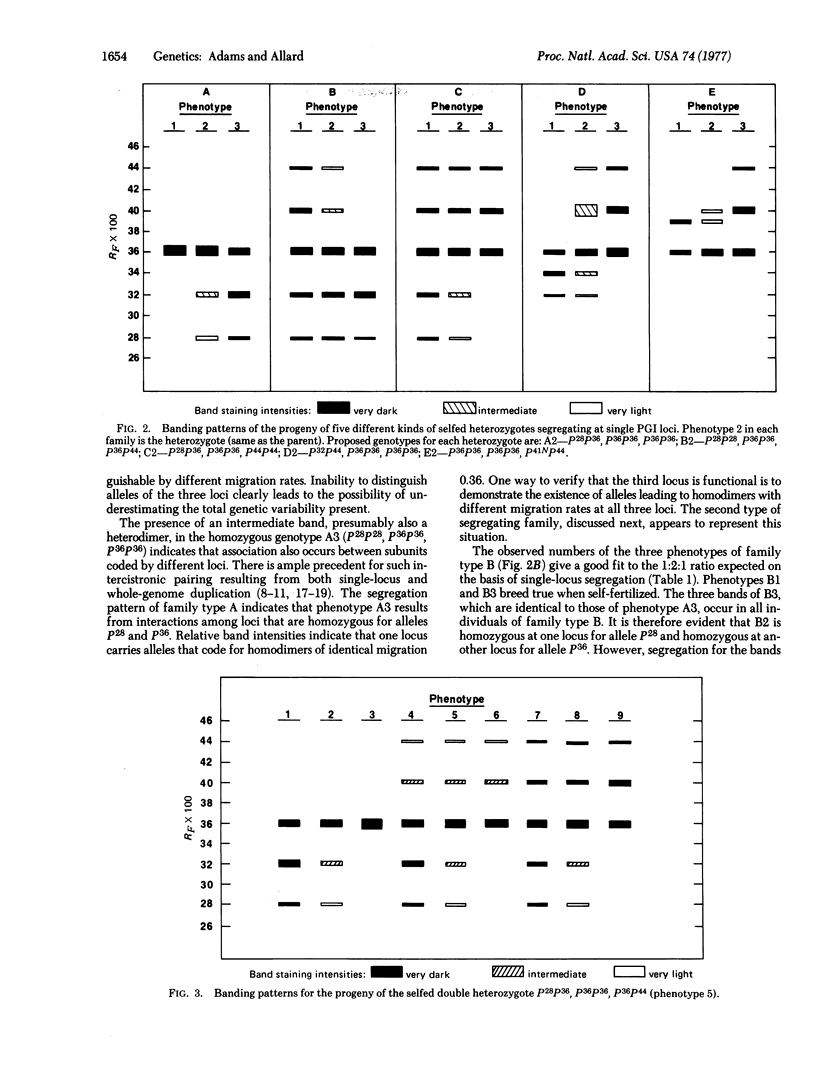
Studies of the inheritance of electrophoretic banding patterns in Festuca microstachys support the hypothesis that three closely related loci, one located in each of the three ancestral genomes, code the multiple phosphoglucose isomerase (glucosephosphate isomerase; D-glucose-6-phosphate ketol-isomerase, EC 5.3.1.9) variants found in this hexaploid species. The close relationship among the three loci is indicated by the observation that hybrid enzymes of intercistronic origin form when the loci in different genomes carry alleles coding homodimers with unlike migration rates. Homozygous individuals fixed for different alleles in different genomes produce hybrid enzymes and, when self-fertilized, they breed true for isozyme patterns normally found only in the heterozygotes of diploid species. Biochemical diversity due to this "fixed heterozygosity" is high in F. microstachys; although this species is more than 99% self-fertilized the proportion of individuals with at least one heterodimer exceeded 61% in all of the 16 natural populations studied and it exceeded 92% in 11 of the populations. This great biochemical diversity may contribute to the ability of F. microstachys to survive in the wide range of habitats in which it is found over western North America.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avise J. C., Kitto G. B. Phosphoglucose isomerase gene duplication in the bony fishes: an evolutionary history. Biochem Genet. 1973 Feb;8(2):113–132. doi: 10.1007/BF00485540. [DOI] [PubMed] [Google Scholar]
- Brentani R., Brentani M. Messenger ribonucleic acid in the nucleolus. Genetics. 1969;61(1 Suppl):391–399. [PMC free article] [PubMed] [Google Scholar]
- Fincham J. R. Heterozygous advantage as a likely general basis for enzyme polymorphisms. Heredity (Edinb) 1972 Jun;28(3):387–391. doi: 10.1038/hdy.1972.49. [DOI] [PubMed] [Google Scholar]
- Freeling M., Schwartz D. Genetic relationships between the multiple alcohol dehydrogenases of maize. Biochem Genet. 1973 Jan;8(1):27–36. doi: 10.1007/BF00485554. [DOI] [PubMed] [Google Scholar]
- Gottlieb L. D. Gene duplication and fixed heterozygosity for alcohol dehydrogenase in the diploid plant Clarkia franciscana. Proc Natl Acad Sci U S A. 1974 May;71(5):1816–1818. doi: 10.1073/pnas.71.5.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb L. D. Genetic control of glutamate oxaloacetate transaminase isozymes in the diploid plant Stephanomeria exigua and its allotetraploid derivative. Biochem Genet. 1973 May;9(1):97–107. doi: 10.1007/BF00485595. [DOI] [PubMed] [Google Scholar]
- Hart G. E. Evidence for triplicate genes for alcohol dehydrogenase in hexaploid wheat. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1136–1141. doi: 10.1073/pnas.66.4.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G. E. Genetic control of alcohol dehydrogenase isozymes in Triticum dicoccum. Biochem Genet. 1969 Dec;3(6):617–625. doi: 10.1007/BF00485484. [DOI] [PubMed] [Google Scholar]
- Salamini F., Tsai C. Y., Nelson O. E. Multiple forms of glucosephosphate isomerase in maize. Plant Physiol. 1972 Aug;50(2):256–261. doi: 10.1104/pp.50.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouros E. Hybrid molecules and the superiority of the heterozygote. Nature. 1976 Jul 15;262(5565):227–229. doi: 10.1038/262227a0. [DOI] [PubMed] [Google Scholar]


