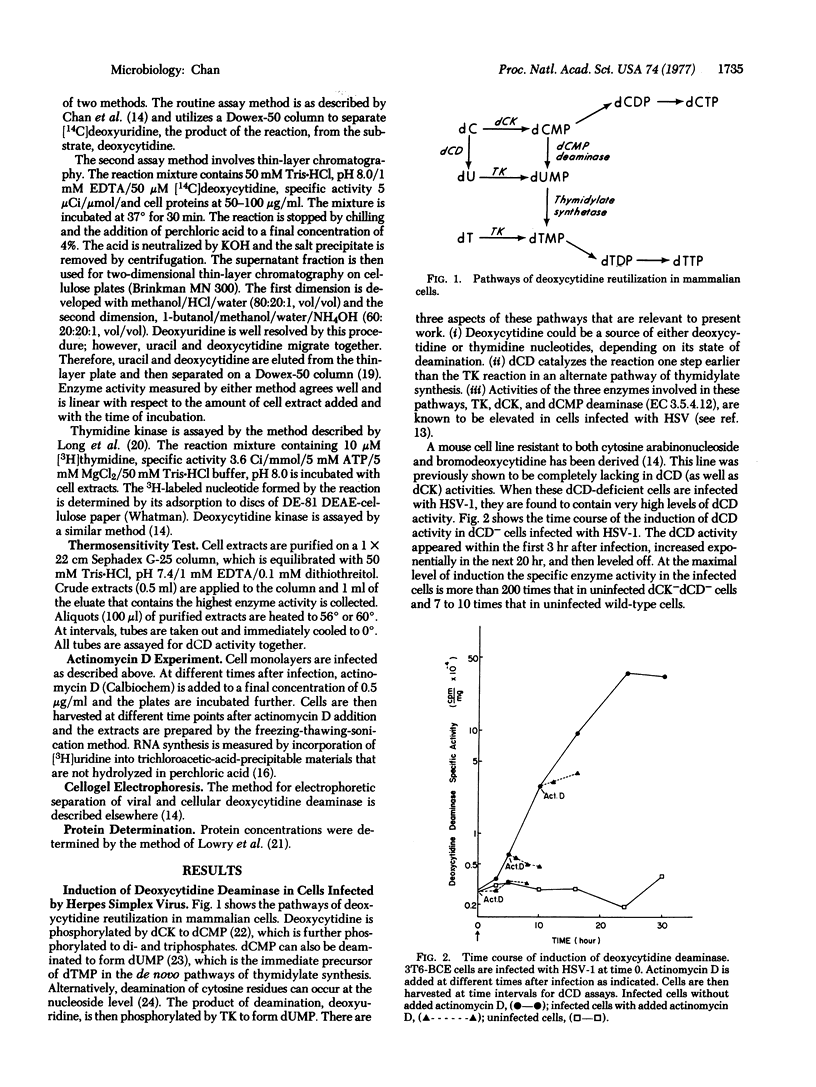
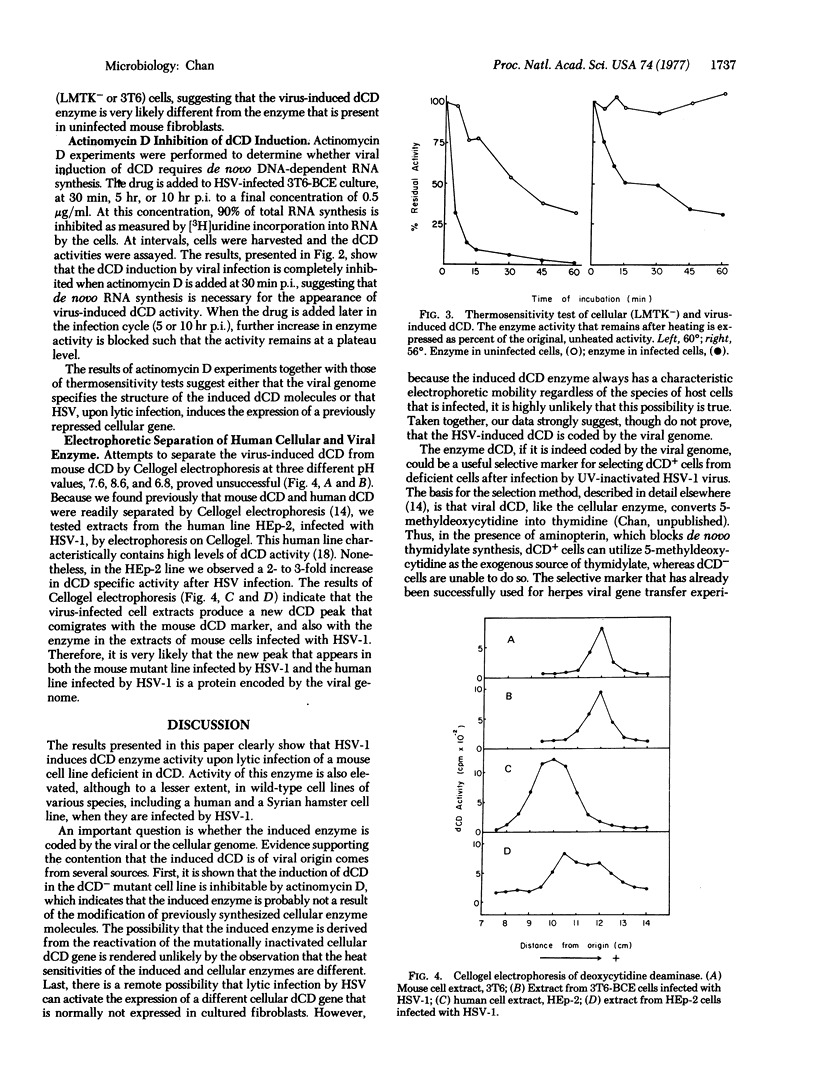
Abstract
Herpes simplex virus type 1 induces deoxycytidine deaminase (cytidine/deoxycytidine aminohydrolase, EC 3.5.4.5) activity when it lytically infects a number of mammalian cell lines. The deaminase activity is induced in a mouse cell line that is deficient in this enzyme. The induction of the enzyme in this mutant cell line does not occur in the presence of actinomycin D and the induced enzyme is more thermolabile than the enzyme of the wild-type mouse cell line. Furthermore, a new deoxycytidine deaminase species with a characteristic electrophoretic mobility that is different from that of the host cell enzyme is found in cell extracts prepared from a human cell line infected with herpesvirus. These results strongly suggest that the virus-induced deoxycytidine deaminase is coded by the viral genome. Because a deficiency in this enzyme is conditionally lethal for cells growing in a medium containing 5-methyldeoxycytidine as the sole source of thymidylate, this enzyme can be utilized as a selective marker for selecting mutant cells that have regained deoxycytidine deaminase activity as the result of infection by ultraviolet-inactivated herpes simplex virus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CREASEY W. A. Studies on the metabolism of 5-iodo-2'-deoxycytidine in vitro. Purification of nucleoside deaminase from mouse kidney. J Biol Chem. 1963 May;238:1772–1776. [PubMed] [Google Scholar]
- Chan T. S., Long C., Green H. A human-mouse somatic hybrid line selected for human deoxycytidine deaminase. Somatic Cell Genet. 1975 Jan;1(1):81–90. doi: 10.1007/BF01538733. [DOI] [PubMed] [Google Scholar]
- Chan T. S., Meuth M., Green H. Pyrimidine excretion by cultured fibroblasts: effect of mutational deficiency in pyrimidine salvage enzymes. J Cell Physiol. 1974 Apr;83(2):263–266. doi: 10.1002/jcp.1040830213. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Goz B., Prusoff W. H. Deoxyribonucleotide metabolism in Herpes simplex virus infected HeLa cells. Biochim Biophys Acta. 1975 May 16;390(3):253–263. doi: 10.1016/0005-2787(75)90346-9. [DOI] [PubMed] [Google Scholar]
- Cooper G. M. Phosphorylation of 5-bromodeoxycytidine in cells infected with herpes simplex virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3788–3792. doi: 10.1073/pnas.70.12.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. MUTANT STRAINS OF HERPES SIMPLEX DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Apr;22:493–502. doi: 10.1016/0042-6822(64)90070-4. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., Adelstein S. J., Oxman M. N. Herpes simplex virus as a source of thymidine kinase for thymidine kinase-deficient mouse cells: suppression and reactivation of the viral enzyme. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1912–1916. doi: 10.1073/pnas.70.7.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. B., Kingsbury D. T. Quantitation of the viral DNA present in cells transformed by UV-irradiated herpes simplex virus. J Virol. 1976 Mar;17(3):788–793. doi: 10.1128/jvi.17.3.788-793.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham J. P., Ives D. H. Deoxycytidine kinase. II. Purification and general properties of the calf thymus enzyme. J Biol Chem. 1970 May 10;245(9):2276–2284. [PubMed] [Google Scholar]
- Garfinkle B., McAuslan B. R. Transformation of cultured mammalian cells by viable herpes simplex virus subtypes 1 and 2. Proc Natl Acad Sci U S A. 1974 Jan;71(1):220–224. doi: 10.1073/pnas.71.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Del Giudice R., Long C. Adenine formation from adenosine by mycoplasmas: adenosine phosphorylase activity. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1401–1405. doi: 10.1073/pnas.72.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A. T., Bjursell G. Deoxyribonucleoside triphosphate pools in herpes simplex type 1 infected cells. J Gen Virol. 1976 Apr;31(1):101–113. doi: 10.1099/0022-1317-31-1-101. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Subak-Sharpe J. H. Biochemical studies on the herpes simplex virus-specified deoxypyrimidine kinase activity. J Gen Virol. 1974 Sep;24(3):481–492. doi: 10.1099/0022-1317-24-3-481. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. Acquisition of thymidine kinase activity by herpes simplex-infected mouse fibroblast cells. Biochem Biophys Res Commun. 1963 Apr 2;11:55–59. doi: 10.1016/0006-291x(63)90027-5. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Anken M. Altered properties of thymidine kinase after infection of mouse fibroblast cells with herpes simplex virus. J Virol. 1967 Feb;1(1):238–240. doi: 10.1128/jvi.1.1.238-240.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemperer H. G., Haynes G. R., Shedden W. I., Watson D. H. A virus-specific thymidine kinase in BHK-21 cells infected with herpes simplex virus. Virology. 1967 Jan;31(1):120–128. doi: 10.1016/0042-6822(67)90015-3. [DOI] [PubMed] [Google Scholar]
- Kraiselburd E., Gage L. P., Weissbach A. Presence of a herpes simplex virus DNA fragment in an L cell clone obtained after infection with irradiated herpes simplex virus I. J Mol Biol. 1975 Oct 5;97(4):533–542. doi: 10.1016/s0022-2836(75)80057-x. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leung W. C., Dubbs D. R., Trkula D., Kit S. Mitochondrial and herpesvirus-specific deoxypyrimidine kinases. J Virol. 1975 Sep;16(3):486–497. doi: 10.1128/jvi.16.3.486-497.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C., Chan T., Levytska V., Kusano T., Green H. Absence of demonstrable linkage of human genes for enzymes of the purine and pyrimidine salvage pathways in human-mouse somatic cell hybrids. Biochem Genet. 1973 Jul;9(3):283–297. doi: 10.1007/BF00485741. [DOI] [PubMed] [Google Scholar]
- McFerran N. V., Smyth M., Orsi B. A. Regulation of cytidine aminohydrolase. Biochem J. 1969 Aug;114(1):8P–9P. doi: 10.1042/bj1140008pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers R., Malathi V. G., Cox R. P., Silber R. Studies on nucleoside deaminase. Increase in activity in HeLa cell cultures caused by cytosine arabinoside. J Biol Chem. 1973 Sep 10;248(17):5909–5913. [PubMed] [Google Scholar]
- Munyon W., Buchsbaum R., Paoletti E., Mann J., Kraiselburd E., Davis D. Electrophoresis of thymidine kinase activity synthesized by cells transformed by herpes simplex virus. Virology. 1972 Sep;49(3):683–689. doi: 10.1016/0042-6822(72)90525-9. [DOI] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil G. L., Moxley T. E., Manak R. C. Enhancement by tetrahydrouridine of 1-beta-D-arabinofuranosylcytosine (cytarabine) oral activity in L1210 leukemic mice. Cancer Res. 1970 Aug;30(8):2166–2172. [PubMed] [Google Scholar]
- Roller B., Cohen G. H. Deoxyribonucleoside triphosphate pools in synchronized human cells infected with herpes simplex virus types 1 and 2. J Virol. 1976 Apr;18(1):58–64. doi: 10.1128/jvi.18.1.58-64.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Summers W. P., Wagner M., Summers W. C. Possible peptide chain termination mutants in thymide kinase gene of a mammalian virus, herpes simplex virus. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4081–4084. doi: 10.1073/pnas.72.10.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]


