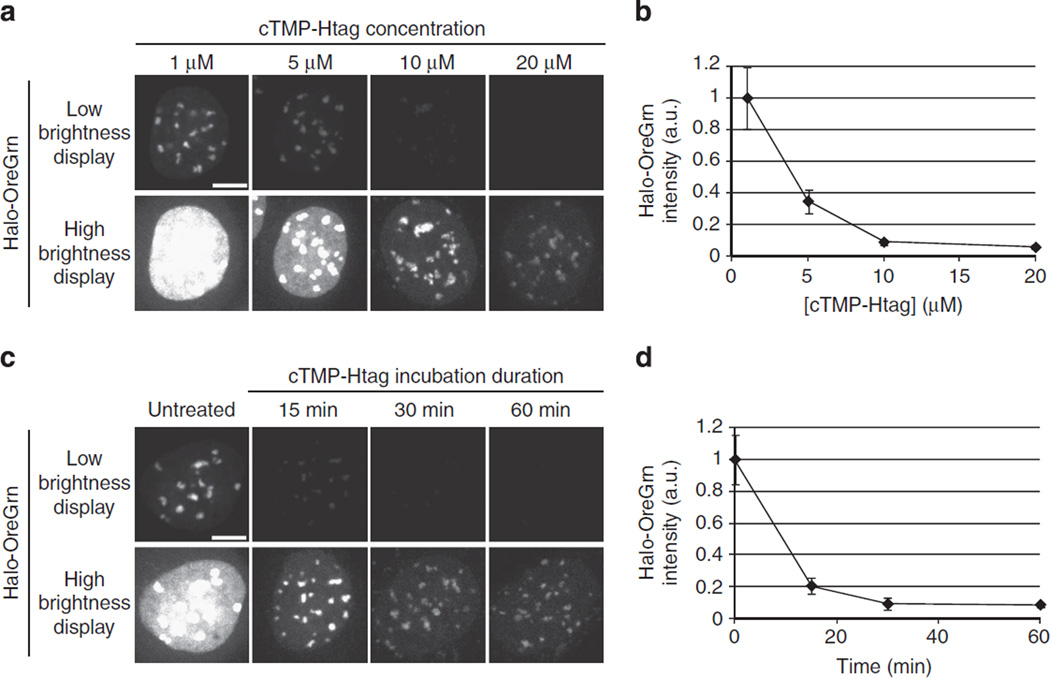
Figure 3. cTMP-Htag enters living cells.
The extent of cTMP-Htag reaction with CENPB-Haloenzyme as a function of cTMP-Htag concentration or treatment time was measured using a dye-blocking assay. Cells expressing CENPB-Haloenzyme (without GFP) were incubated with cTMP-Htag, washed, treated with 100nM Halotag-Oregon Green (Halo-OreGrn) for 20min, then washed again before imaging. Decreased Halo-OreGrn indicates cTMP-Htag occupancy of CENPB-Haloenzyme sites. Each Halo-OreGrn image is displayed at two brightness levels to aid visualization. Within each row, all the images are displayed using identical brightness levels. (a,b) Cells were treated with 1, 5, 10 or 20 mM cTMP-Htag for 1 h, then treated with Halo-OreGrn as described above. Treatment with 10 µM cTMP-Htag for 1 h is sufficient to block ~90% of Halo-OreGrn binding. (c,d) Cells were treated with 20 µM cTMP-Htag for 15, 30 or 60min, or left untreated as control, then treated with Halo-OreGrn as described above. Treatment with 20 µM cTMP-Htag for 30min is sufficient to block ~90% of Halo-OreGrn binding. Images (a,c) are maximum-intensity projections of representative cells from each condition. Average Halo-OreGrn intensity at centromeres was quantified for each condition (b,d). Error bars represent s.d. (n≥15 fields for each data point, multiple cells per field). a.u., arbitrary unit.