Table 3. Decarboxylative Olefination: Carboxylic Acid Scopea.
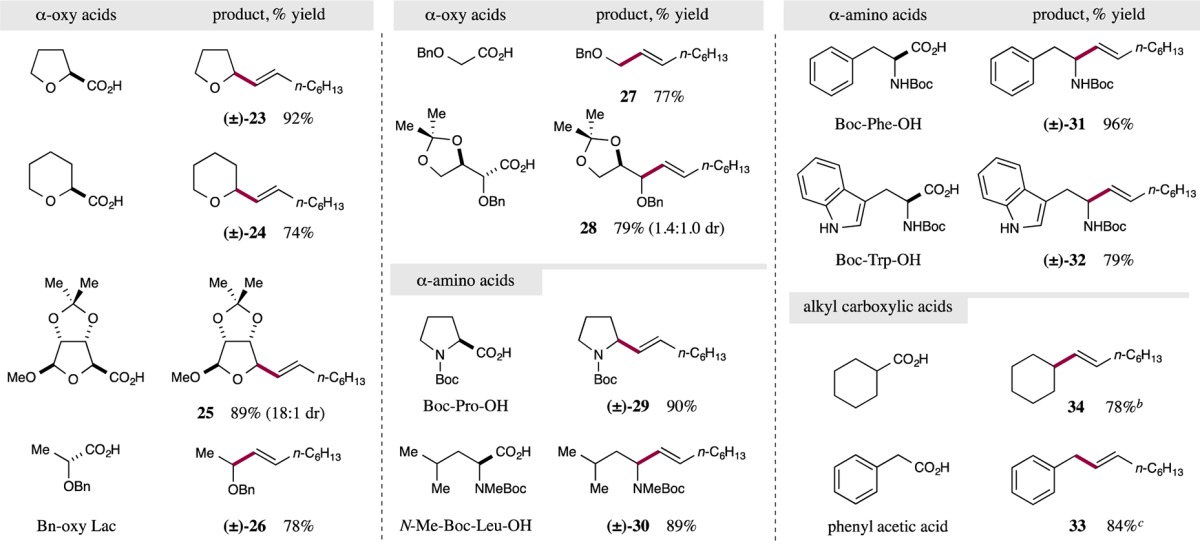
Reactions performed using the optimized conditions from Table 1 with 0.5 mmol (E)-1-iodo-1-octene or (E)-1-bromo-1-octene. Yields are of isolated products. Ratios of diastereomers determined by 1H NMR analysis. For detailed experimental procedures, see SI.
Primary alkyl carboxylic acids provided encouraging levels of efficiency, see SI. for experimental results.
Good yields were obtained for phenyl acetic acid derivatives p-OMe (96% yield) and m-Cl (68% yield). For experimental procedures, see SI.
