Abstract
Adult BALB/c mice, individually housed, were stimulated with nine different stressors, arranged randomly, for 4 continuous weeks to generate an animal model of chronic stress. In chronically stressed mice, spontaneous locomotor activity was significantly decreased, escape latency in the Morris water maze test was prolonged, serum levels of total thyrotropin and total triiodothyronine were significantly decreased, and dopamine and norepinephrine content in the pallium, hippocampus and hypothalamus were significantly reduced. All of these changes were suppressed, to varying degrees, by L-tyrosine supplementation. These findings indicate that the neuroendocrine network plays an important role in chronic stress, and that L-tyrosine supplementation has therapeutic effects.
Keywords: chronic unpredictable stress, neuroendocrine network, total thyrotropin, total triiodothyronine, dopamine, norepinephrine, L-tyrosine, neural regeneration
Abbreviations
CUS, chronic and unpredictable stress group; TT4, total triiodothyronine; TT3, total thyrotropin; TRH, thyrotropin-releasing hormone; TSH, thyroid-stimulating hormone
INTRODUCTION
Stress is the body's non-specific response to both internal and external stimuli. Persistent or excessive stress, surpassing the individual's capacity to adapt and cope, disturbs normal physiological function and behavior, even leading to physical and psychiatric diseases[1,2,3]. Statistics show that the stress response can cause histological, physiological and biochemical changes[4,5,6]. As the pace of social life increases and competition intensifies, people generally are in a state of chronic stress that can give rise to a series of familial and social problems. Therefore, it is an urgent task for modern medicine to study relevant mechanism of stress, and to investigate methods to alleviate its impact on human health. Tyrosine, an aromatic amino acid, is a precursor for the synthesis of catecholamine neurotransmitters[7]. In the central nervous system, catecholamine neurotransmitters participate in regulating activities such as awakening, attention and mood[8]. Therefore, when catecholamine insufficiency arises in the brain due to chronic stress, supplementation with tyrosine can reduce physical decline, loss of interest and slow movement caused by chronic stress[9,10]. Previous studies indicate that tyrosine plays an active part in regulating the behavior and mood of people in such extreme environments as high altitude, oxygen deficiency and extreme cold[11,12,13]. However, little is known regarding the effects of tyrosine on disorders resulting from daily stresses (e.g., the quickened pace of life, fiercer social competition and increasing pressure at work, the disparity between efforts and gains, occupational instability and interpersonal tension). In the present study, we established a mouse model of chronic stress to investigate the effects of tyrosine intervention on disruption of the neuroendocrine network caused by chronic stress, and to clarify the underlying mechanism.
RESULTS
Quantitative analysis of experimental animals
A total of 63 healthy BALB/c mice were randomly divided into three groups with 21 mice in each group: normal control (control), chronic and unpredictable stress (CUS) and CUS plus L-tyrosine interference group (CUS-L). Experiments lasted for 4 weeks. All mice were included in the final analysis with no cases of death or infection.
Stress increased mouse body weight
After 4 weeks of chronic and unpredictable stress, the body weight of mice increased significantly compared with that 4 weeks earlier (P < 0.05; Table 1).
Table 1.
Effect of chronic stress on body weight (g) of mice
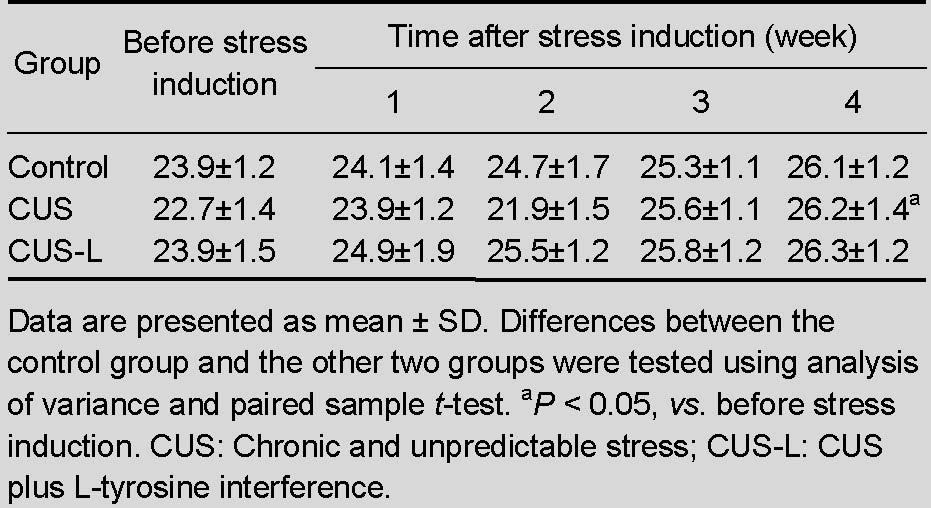
L-tyrosine alleviated behavioral deficits in stressed mice
Spontaneous locomotor activity
Compared with normal control mice, stressed mice displayed a significant reduction in the total horizontal distance traveled in a novel environment (P < 0.01). L-tyrosine supplementation significantly increased the total horizontal distance traveled by mice subjected to stress (P < 0.01; Table 2).
Table 2.
Locomotor activity (m) of mice in each group after exposure to chronic stress
The Morris water maze test
After five successive tests in 4 weeks, the time taken by the control mice to find the platform gradually decreased, while mice submitted to stress showed the opposite trend, and there was a significant difference between these two groups (P < 0.05). Stressed mice given L-tyrosine supplementation showed greater improvement in performance in maze tests (P < 0.05; Figure 1).
Figure 1.
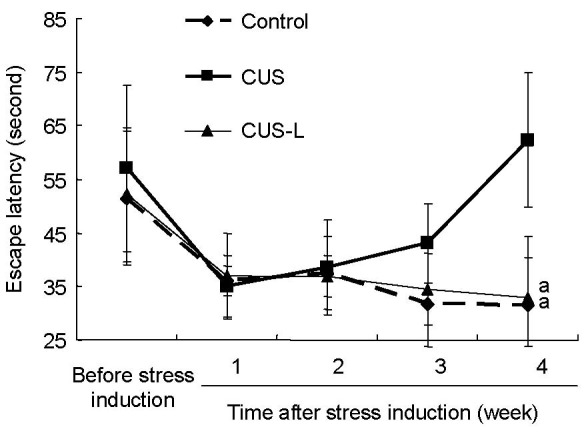
Escape latency trends at 1–4 weeks in the Morris water maze test.
Data are presented as mean ± SD. Differences between the control group and the other two groups were tested using analysis of variance and paired sample t-test. aP < 0.05, vs. CUS group. CUS: Chronic and unpredictable stress; CUS-L: CUS plus L-tyrosine interference.
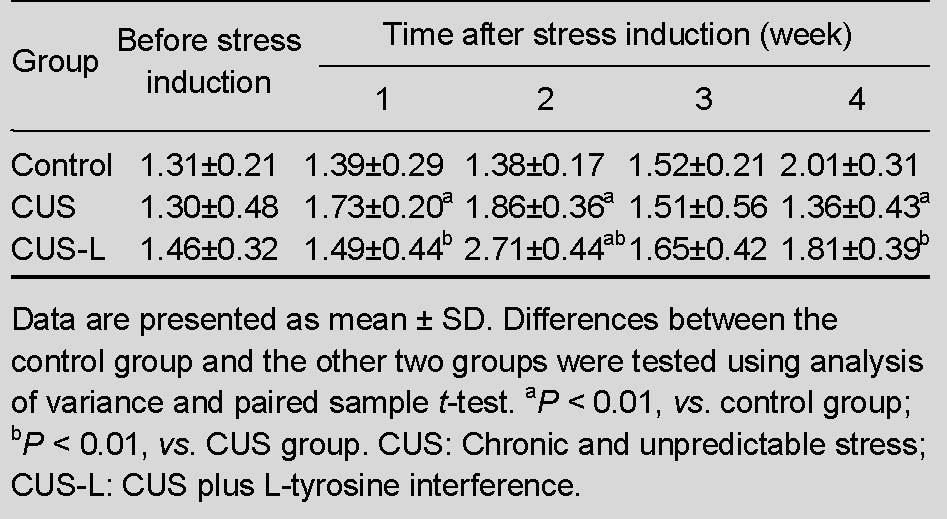
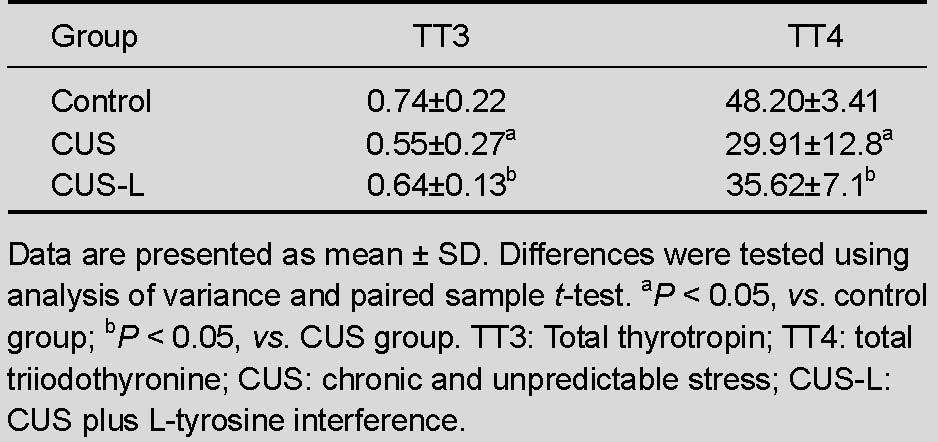
L-tyrosine increased serum levels of total thyrotropin (TT3) and total triiodothyronine (TT4) in stressed mice
Compared with mice in the control group, serum levels of TT3 and TT4 were significantly decreased in stressed mice (P < 0.05). TT3 and TT4 serum levels were significantly increased by L-tyrosine supplementation (P < 0.05; Table 3).
Table 3.
Effect of chronic stress on serum levels of TT3 and TT4 (ng/mL)
L-tyrosine supplementation modulated neurochemical changes in brain tissues of stressed mice
After 4 weeks of chronic stress, dopamine and norepinephrine levels in the pallium, hippocampus and hypothalamus were significantly decreased (P < 0.01 or P < 0.05). Levels of dopamine in the pallium and hippocampus, as well as levels of norepinephrine in the pallium and hypothalamus, were restored by L-tyrosine supplementation (P < 0.05 or P < 0.01; Figure 2).
Figure 2.
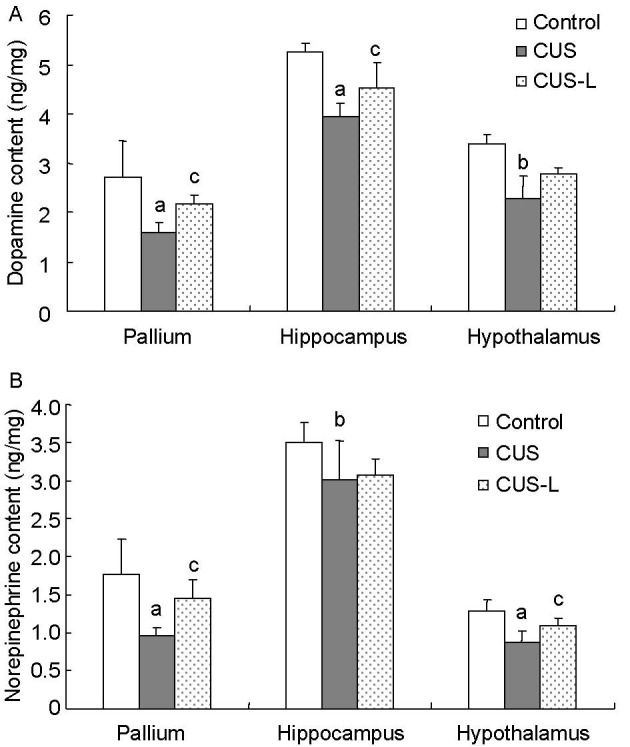
Dopamine (A) and norepinephrine (B) content in the pallium, hippocampus and hypothalamus (ng/mg).
Data are presented as mean ± SD. Differences between the control group and the other two groups were tested using analysis of variance and paired sample t-test. aP < 0.01 and bP < 0.05, vs. control group; cP < 0.05, vs. CUS group.
CUS: Chronic and unpredictable stress; CUS-L: CUS plus L-tyrosine interference.
DISCUSSION
In daily life, chronic stress-related hazards are generally caused by various complex, low-intensity and long-term life events[14]. Single stress factors (e.g., amputation of the tail, high-frequency-voltage convulsion and long-term restraint) were, in the past, frequently used to reproduce stress in animal models in a relatively short period of time[15]. However, single stress factors do not fully reproduce chronic stress because they are too intense or animals develop resistance easily[16]. While multiple chronic and unpredictable factors are better able to reproduce chronic stress in animal models, this approach has its own shortcomings, including a lengthy model production period, high demands on laboratory resources and the requirement to frequently change stimulants, which results in a heavy workload for the researcher[17,18].
After 4 weeks of chronic stress, the body weight of mice in the CUS group showed a significant increase compared with that before the experiment. In previous studies, some mice suffered a loss of weight, while some gained weight. Thus, our result is not contradictory to those previously reported by various groups[15,19,20]. The distance covered per unit time by CUS mice in the first and second weeks of testing increased gradually, but their performance declined in the spontaneous movement experiment from the third week. This indicates that damage caused by chronic stress to physical and psychological health begins to manifest from the third week, and that chronic stress can weaken the animal physically, leading to increased fatigability, which hinders their ability to explore the surrounding environment in a limited time. The increased escape latency of the stressed mice suggests that the animals’ learning and memory abilities decline with increasing duration of stress, and that chronic stress may damage their learning and memory functions.
Rodents’ memory ability is related to prefrontal cortex dopamine content, and proper functioning of the dopaminergic system is critical to good working memory[21,22,23,24]. Studies show that the locus coeruleus releases substantial amounts of norepinephrine during awakening, decreases release significantly in the period of slow-wave sleep, and completely halts the release of the neurotransmitter in the period of paradoxical sleep, which causes people not to remember their dreams. This indicates that norepinephrine regulates the plasticity of nerve cells and promotes memory[25,26,27,28]. In this experiment, supplementation with tyrosine increased levels of catecholamine neurotransmitters in the brain, thereby improving the learning and memory abilities of the mice.
The lowered serum TT3 and TT4 levels in the stressed mice clearly show that the hypothalamus-hypophysis- thyroid axis plays an important role in the stress response. Under stress, reduced activities of thyroid peroxidase and oxidase, which are necessary for TT3 synthesis, leads to decreased serum levels of TT3 in the peripheral blood and quicker transformation of TT4 into the non-bioactive TT3. Central nervous system norepinephrine functions to inhibit the hypophysis-adrenal cortex axis and activate the hypophysis-thyroid axis, both of which affect thyroid function, which depends on the regulation of the hypothalamus-hypophysis-thyroid axis. Other central nervous system regions act on the arcuate nucleus in the center of the hypothalamus, causing it to produce and release thyrotropin-releasing hormone, which induces anterior pituitary cells to secrete thyroid-stimulating hormone, which regulates thyroid hormone levels[29,30,31,32]. Normal levels of thyroid hormone help regulate energy metabolism in the body, quicken the repair and the renewal of damaged cells, and strengthen resistance to chronic stress. Clinically, many patients with depression caused by social stress present with weakened immunity and easy fatigability due to decreased serum levels of TT3 and TT4. Supplementation with tyrosine can increase norepinephrine in the brain and induce thyrotropin-releasing hormone neurons to release more thyrotropin-releasing hormone, which acts on the hypophysis to release more thyroid-stimulating hormone. As a result, the synthesis and release of thyroid hormones increase[33,34,35].
In summary, chronic stress causes behavioral changes and disorders in the neuroendocrine network, and L-tyrosine can relieve or inhibit these changes. However, the stress response itself is dynamic, and examining only one specific time point cannot fully clarify the complexities of this process.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The experiments were performed at a laboratory in the Department of Pathophysiology of Peking Union Medical College in China from March 2008 to September 2010.
Materials
Animals
Sixty-three genetically similar male BALB/c mice, weighing 23-24 g and 8-9 weeks of age, were provided by the Experimental Animal Center, Peking Union Medical College in China (certification No. SYXK (Jing) 2008-0098). The animals were housed at a controlled temperature (22 ± 2°C) and humidity (45-65%) in artificially lighted rooms with a 12-hour light/dark cycle (lights on at 7: 00 a.m.), with free access to food and water. During the first week after arrival, animals were allowed to habituate to their new environment without handling. The experiments were performed in a different room with the same temperature.
Drugs
L-tyrosine (Sigma, St. Louis, MO, USA) was dissolved in sterile 0.9% saline[36].
Methods
Stress induction
Mice in the CUS and CUS-L groups, which were housed alone, were subjected to nine types of stressors, including cage tilting (45 °, 12 hours), an empty cage with water on the bottom (12 hours), inversion of the light/dark cycle (12 hours), vibration (45 minutes), immersion in water (4°C, 5 minutes), restraint (2 hours), food deprivation (12 hours), water deprivation with empty water bottles (12 hours) and unpredictable 1-second foot shocks (0.3 mA). Two of these stressors were applied daily in a random manner to ensure that mice could not predict which type of stress would be presented[37,38,39]. The mice in the control group were housed together (21 mice in one cage) and did not receive any stimulation. The weights of the mice were monitored before experiments every week
L-tyrosine administration
Mice from the CUS-L group (L-tyrosine suspension, 5 mL, 2.032 g/kg), control group and CUS group (distilled water, the same volume as for the CUS-L group) received intragastric administration of the appropriate solution daily before stress induction (lasting for 28 successive days)[36].
Behavioral observations
The stress procedure lasted for 4 weeks, and behavioral testing was performed at the end of every week.
Spontaneous locomotor activity
Each mouse was individually placed in an automated locomotor activity chamber (50 × 50 × 40 cm3) equipped with horizontal and vertical infrared beams (Institute of Materia Medica, Peking Union Medical College, Beijing, China). The chambers were placed in a dimly lit room illuminated by four overhead 15 V projection lamps mounted 200 cm above the chambers. The animal was placed in the chamber and allowed to move spontaneously for 120 seconds (18:00 and 21:00). The total horizontal activity distance (m) traveled by mice in the test chamber was recorded with a video camera placed above the chamber and analyzed with DigBehv software (Version 2.0; Shanghai Jiliang Software Technology Co., Ltd., Shanghai, China). Data were collected before the stress was applied and on the last day of every week[40,41,42].
The Morris water maze test
The Morris water maze test was conducted in a large circular pool with a diameter of 80 cm and four side walls approximately 70 cm high, containing no internal cues, stimuli, markings or objects, but surrounded by stable, salient extra-maze cues (Institute of Materia Medica, Peking Union Medical College, China). The pool was filled with water at a temperature of 22 ± 1°C to a depth of about 35 cm. The pool was conceptually divided into four quadrants with equal areas (NE, SE, SW and NW). A circular escape platform (8 cm in diameter and 34 cm in height) was placed into the tank at a fixed position in the center of the NW quadrant, and was 20 cm away from the wall. Black nontoxic carbon ink (Chinese ink) was added to the pool to make the water opaque. The top surface of the platform was 1 cm below the water surface, with a rough surface to make it easy for the mice to climb on. The test was given before the stress had been applied and was conducted on the last day of every week between 21:00 and 23:00. During the test trial, the mouse was released into the water at one of the three different starting positions (in three different quadrants that did not contain the platform) with its head facing the wall. And then the mouse was allowed to swim for 120 seconds to search for the hidden platform. If it failed to locate the platform within 120 seconds, escape would be assisted and escape latency was recorded as 120 seconds. At the end of each trial, each mouse would stay on the platform for 3 seconds. The sequence of the starting positions remained the same for all the mice within one session, but changed each session. During each trial session, escape latency (the time taken to find the platform) was recorded by a computerized video imaging analysis system[43,44,45,46,47] (supplementary Figure 1 online).
Sampling
Mice were sacrificed by cervical dislocation at the end of the last behavioral tests, and serum and brain tissues (pallium, hippocampus and hypothalamus[48,49]) were immediately collected and stored at -80°C for experiments.
Determination of serum levels of thyroid hormone
Thyroid hormone levels were analyzed using radioimmunoassay kits with 125I as a tracer (China Institute of Atomic Energy, Beijing, China). All assays were performed in duplicate according to the manufacturer's instructions. Analyses were conducted at room temperature on 50-μL samples for TT4 and TT3. The reaction system was composed of standard (50 μL, 0, 0.5, 1, 2, 4, 8 ng/mL), samples (50 μL) and 125I tracer (200 μL), each of which was at an appropriate volume. After incubation, the reaction system was separated by adding a separation reagent (500 μL), except for the tubes set to measure total counts, and centrifuged at 4 000 r/min for 25 minutes. The supernatant was decanted, and the radioactivity of the pellet was counted with a gamma counter (GAMMA-C12, DPC, USA)[50,51].
Measurement of catecholamines by high performance liquid chromatography
Assays for norepinephrine and dopamine were performed with high performance liquid chromatography. The pallium, hippocampus and hypothalamus were sonicated in 1.5 mL 0.1 M HClO4 and 40 μL 3,4-dihydroxy-benzylamine as the internal standard and centrifuged at 14 000 r/m for 15 minutes. Supernatants (20 μL) were injected onto a 4.5 mm × 250 mm, 10 μm chromatography column (Shimadzu, Japan) in a mobile phase containing KH2PO4 (100 mM), sodium 1-octanesulfonate (1.0 mM), ethylenediamine tetraacetic acid-Na2 (0.5 mM), methanol (11% v/v) and pure water. Sample amounts were calculated by comparing the relative peak areas of sample peaks to internal standards. Norepinephrine and dopamine levels were measured in a single chromatogram. Concentrations were expressed as nanogram of norepinephrine or dopamine per milligram of sample tissue wet weight[52,53,54,55].
Statistical analysis
Data were analyzed using SPSS version 17.0 (SPSS, Chicago, IL, USA) and were expressed as mean ± SD. One-way analysis of variance was performed for intergroup comparisons for the same rearing condition at different time points. The paired sample t-test was used for intergroup comparisons for different rearing conditions. A value of P < 0.05 was considered statistically significant.
Acknowledgments
We would like to thank the laboratory of the Department of Pathophysiology of Peking Union Medical College in China for the experimental equipment and space.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 30370537.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee of Bethune International Peace Hospital of Chinese PLA, China.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org.
(Yu YQ, He ZY/Song LP)
REFERENCES
- [1].Grewen KM, Anderson BJ, Girdler SS, et al. Warm partner contact is related to lower cardiovascular reactivity. Behav Med. 2003;29(3):123–130. doi: 10.1080/08964280309596065. [DOI] [PubMed] [Google Scholar]
- [2].Zhu GJ, Duan YP, Xue QF, et al. Changes of urine and plasam catecholmine of expendition members in antartic environment. Jidi Yanjiu. 1998;10(4):305–309. [Google Scholar]
- [3].Xu C, Zhu G, Xue Q, et al. Effect of the Antarctic environment on hormone levels and mood of Chinese expeditioners. Int J Circumpolar Health. 2003;62(3):255–267. doi: 10.3402/ijch.v62i3.17562. [DOI] [PubMed] [Google Scholar]
- [4].Quan M, Zheng C, Zhang N, et al. Impairments of behavior, information flow between thalamus and cortex, and prefrontal cortical synaptic plasticity in an animal model of depression. Brain Res Bull. 2011;85(3-4):109–116. doi: 10.1016/j.brainresbull.2011.03.002. [DOI] [PubMed] [Google Scholar]
- [5].Shahzad MM, Arevalo JM, Armaiz-Pena GN, et al. Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J Biol Chem. 2010;285(46):35462–35470. doi: 10.1074/jbc.M110.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Venditti P, Napolitano G, Di Stefano L, et al. Effect of vitamin E administration on response to ischemia- reperfusion of hearts from cold-exposed rats. Exp Physiol. 2011;96(7):635–646. doi: 10.1113/expphysiol.2011.058289. [DOI] [PubMed] [Google Scholar]
- [7].McCann UD, Penetar DM, Shaham Y, et al. Effects of catecholamine depletion on alertness and mood in rested and sleep deprived normal volunteers. Neuropsychopharmacology. 1993;8(4):345–356. doi: 10.1038/npp.1993.34. [DOI] [PubMed] [Google Scholar]
- [8].Klimek DL, Cruz OA, Scott WE, et al. Isoametropic amblyopia due to high hyperopia in children. J AAPOS. 2004;8(4):310–313. doi: 10.1016/j.jaapos.2004.05.007. [DOI] [PubMed] [Google Scholar]
- [9].Goddard AW, Ball SG, Martinez J, et al. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety. 2010;27(4):339–350. doi: 10.1002/da.20642. [DOI] [PubMed] [Google Scholar]
- [10].Bondi CO, Jett JD, Morilak DA. Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by alpha1-adrenergic receptors in medial prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):913–923. doi: 10.1016/j.pnpbp.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li ZM, Xu CL, Zu SY. 3. Vol. 24. Guowai Yixue: Shengli Bingli Yixue yu Linchuang Fence; 2004. The role of sympathetic nerves in immune suppression of chronic stress; pp. 53–58. [Google Scholar]
- [12].Haney M, Maccari S, Le Moal M, et al. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698(1-2):46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- [13].Rodriguez M, Santana C, Afonso D. Maternal ingestion of tyrosine during rat pregnancy modifies the offspring behavioral lateralization. Physiol Behav. 1994;55(4):607–613. doi: 10.1016/0031-9384(94)90033-7. [DOI] [PubMed] [Google Scholar]
- [14].Wei L, David A, Duman RS, et al. Early life stress increases anxiety-like behavior in Balbc mice despite a compensatory increase in levels of postnatal maternal care. Horm Behav. 2010;57(4-5):396–404. doi: 10.1016/j.yhbeh.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nativio P, Pascale E, Maffei A, et al. Effect of stress on hippocampal nociceptin expression in rat. Stress. doi: 10.3109/10253890.2011.627071. in press. [DOI] [PubMed] [Google Scholar]
- [16].Ricon T, Toth E, Leshem M, et al. Unpredictable chronic stress in juvenile or adult rats has opposite effects, respectively, promoting and impairing resilience. Stress. 2012;15(1):11–20. doi: 10.3109/10253890.2011.572207. [DOI] [PubMed] [Google Scholar]
- [17].Dal-Zotto S, Martí O, Armario A. Influence of single or repeated experience of rats with forced swimming on behavioural and physiological responses to the stressor. Behav Brain Res. 2000;114(1-2):175–181. doi: 10.1016/s0166-4328(00)00220-5. [DOI] [PubMed] [Google Scholar]
- [18].Alcaro A, Cabib S, Ventura R, et al. Genotype- and experience-dependent susceptibility to depressive-like responses in the forced-swimming test. Psychopharmacology (Berl) 2002;164(2):138–143. doi: 10.1007/s00213-002-1161-8. [DOI] [PubMed] [Google Scholar]
- [19].Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134(4):319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- [20].Martin JV, Edwards E, Johnson JO, et al. Monoamine receptors in an animal model of affective disorder. J Neurochem. 1990;55(4):1142–1148. doi: 10.1111/j.1471-4159.1990.tb03117.x. [DOI] [PubMed] [Google Scholar]
- [21].Roozendaal B, Hahn EL, Nathan SV, et al. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J Neurosci. 2004;24(37):8161–8169. doi: 10.1523/JNEUROSCI.2574-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Thierry AM, Tassin JP, Blanc G, et al. Selective activation of mesocortical DA system by stress. Nature. 1976;263(5574):242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- [23].Donnelly E. Work-related stress and posttraumatic stress in emergency medical services. Prehosp Emerg Care. 2012;16(1):76–85. doi: 10.3109/10903127.2011.621044. [DOI] [PubMed] [Google Scholar]
- [24].Palanisamy A, Baxter MG, Keel PK, et al. Rats exposed to isoflurane in utero during early gestation are behaviorally abnormal as adults. Anesthesiology. 2011;114(3):521–528. doi: 10.1097/ALN.0b013e318209aa71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cirelli C. How sleep deprivation affects gene expression in the brain: a review of recent findings. J Appl Physiol. 2002;92(1):394–400. doi: 10.1152/jappl.2002.92.1.394. [DOI] [PubMed] [Google Scholar]
- [26].Cotella EM, Lascano IM, Levin GM, et al. Amitriptyline treatment under chronic stress conditions: effect on circulating catecholamines and anxiety in early maternally separated rats. Int J Neurosci. 2009;119(5):664–680. doi: 10.1080/00207450802330611. [DOI] [PubMed] [Google Scholar]
- [27].Uygur EE, Arslan M. Effects of chronic stress on cognitive functions and anxiety related behaviors in rats. Acta Physiol Hung. 2010;97(3):297–306. doi: 10.1556/APhysiol.97.2010.3.6. [DOI] [PubMed] [Google Scholar]
- [28].Hoffman AN, Krigbaum A, Ortiz JB, et al. Recovery after chronic stress within spatial reference and working memory domains: correspondence with hippocampal morphology. Eur J Neurosci. 2011;34(6):1023–1030. doi: 10.1111/j.1460-9568.2011.07820.x. [DOI] [PubMed] [Google Scholar]
- [29].Fuxe K, Corrodi H, Hökfelt T, et al. Central monoamine neurons and pituitary-adrenal activity. Prog Brain Res. 1970;32:42–56. doi: 10.1016/S0079-6123(08)61518-6. [DOI] [PubMed] [Google Scholar]
- [30].Hou TD, Du JZ. Norepinephrine attenuates hypoxia-inhibited thyrotropin-releasing hormone release in median eminence and paraventricular nucleus of rat hypothalamus. Neuro Endocrinol Lett. 2005;26(1):43–49. [PubMed] [Google Scholar]
- [31].Anju TR, Nandhu MS, Jes P, et al. Endocrine regulation of neonatal hypoxia: role of glucose, oxygen, and epinephrine supplementation. Fetal Pediatr Pathol. 2011;30(5):338–349. doi: 10.3109/15513815.2011.587498. [DOI] [PubMed] [Google Scholar]
- [32].Lee SJ, Kang JG, Ryu OH, et al. The relationship of thyroid hormone status with myocardial function in stress cardiomyopathy. Eur J Endocrinol. 2009;160(5):799–806. doi: 10.1530/EJE-08-0808. [DOI] [PubMed] [Google Scholar]
- [33].Kioukia N, Bekris S, Antoniou K, et al. Effects of chronic mild stress (CMS) on thyroid hormone function in two rat strains. Psychoneuroendocrinology. 2000;25(3):247–257. doi: 10.1016/s0306-4530(99)00051-7. [DOI] [PubMed] [Google Scholar]
- [34].Sarkar S, Biswas SC, Chatterjee O, et al. Protein kinase A linked phosphorylation mediates triiodothyronine induced actin gene expression in developing brain. Brain Res Mol Brain Res. 1999;67(1):158–164. doi: 10.1016/s0169-328x(99)00056-x. [DOI] [PubMed] [Google Scholar]
- [35].Helmreich DL, Tylee D. Thyroid hormone regulation by stress and behavioral differences in adult male rats. Horm Behav. 2011;60(3):284–291. doi: 10.1016/j.yhbeh.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lehnert H, Reinstein DK, Strowbridge BW, et al. Neurochemical and behavioral consequences of acute, uncontrollable stress: effects of dietary tyrosine. Brain Res. 1984;303(2):215–223. doi: 10.1016/0006-8993(84)91207-1. [DOI] [PubMed] [Google Scholar]
- [37].Thurmond JB, Freeman GB, Soblosky JS, et al. Effects of dietary tyrosine on L-dopa- and amphetamine-induced changes in locomotor activity and neurochemistry in mice. Pharmacol Biochem Behav. 1990;37(2):259–266. doi: 10.1016/0091-3057(90)90331-b. [DOI] [PubMed] [Google Scholar]
- [38].Overstreet DH. Modeling depression in animal models. Methods Mol Biol. 2012;829:125–144. doi: 10.1007/978-1-61779-458-2_7. [DOI] [PubMed] [Google Scholar]
- [39].Avraham Y, Bonne O, Berry EM. Behavioral and neurochemical alterations caused by diet restriction--the effect of tyrosine administration in mice. Brain Res. 1996;732(1-2):133–144. doi: 10.1016/0006-8993(96)00514-8. [DOI] [PubMed] [Google Scholar]
- [40].Furuse T, Yamada I, Kushida T, et al. Behavioral and neuromorphological characterization of a novel Tuba1 mutant mouse. Behav Brain Res. 2012;227(1):167–174. doi: 10.1016/j.bbr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- [41].Raineri M, Peskin V, Goitia B, et al. Attenuated methamphetamine induced neurotoxicity by modafinil administration in mice. Synapse. 2011;65(10):1087–1098. doi: 10.1002/syn.20943. [DOI] [PubMed] [Google Scholar]
- [42].Dal-Pan A, Pifferi F, Marchal J, et al. Cognitive performances are selectively enhanced during chronic caloric restriction or resveratrol supplementation in a primate. PLoS One. 2011;6(1):e16581. doi: 10.1371/journal.pone.0016581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liu L, Orozco IJ, Planel E, et al. A transgenic rat that develops Alzheimer's disease-like amyloid pathology, deficits in synaptic plasticity and cognitive impairment. Neurobiol Dis. 2008;31(1):46–57. doi: 10.1016/j.nbd.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kukar T, Prescott S, Eriksen JL, et al. Chronic administration of R-flurbiprofen attenuates learning impairments in transgenic amyloid precursor protein mice. BMC Neurosci. 2007;8:54. doi: 10.1186/1471-2202-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chengzhi C, Yan T, Xuejun J, et al. Recovery of chronic noise exposure induced spatial learning and memory deficits in young male Sprague-Dawley rats. J Occup Health. 2011;53(3):157–163. doi: 10.1539/joh.l10125. [DOI] [PubMed] [Google Scholar]
- [46].Li H, Zhang L, Fang Z, et al. Behavioral and neurobiological studies on the male progeny of maternal rats exposed to chronic unpredictable stress before pregnancy. Neurosci Lett. 2010;469(2):278–282. doi: 10.1016/j.neulet.2009.12.017. [DOI] [PubMed] [Google Scholar]
- [47].Hock BJ, Lattal KM, Kulnane LS, et al. Pathology associated memory deficits in Swedish mutant genome- based amyloid precursor protein transgenic mice. Curr Aging Sci. 2009;2(3):205–213. doi: 10.2174/1874609810902030205. [DOI] [PubMed] [Google Scholar]
- [48].Hsin H, Kim MJ, Wang CF, et al. Proline-rich tyrosine kinase 2 regulates hippocampal long-term depression. J Neurosci. 2010;30(36):11983–11993. doi: 10.1523/JNEUROSCI.1029-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lu H, Pang W, Hu YD, et al. Effects of intracellular zinc depletion on the expression of VDAC in cultured hippocampal neurons. Nutr Neurosci. 2011;14(2):80–87. doi: 10.1179/1476830511Y.0000000004. [DOI] [PubMed] [Google Scholar]
- [50].Thevarajah M, Chew YY, Lim SC, et al. Determination of trimester specific reference intervals for thyroid hormones during pregnancy in Malaysian women. Malays J Pathol. 2009;31(1):23–27. [PubMed] [Google Scholar]
- [51].Dhatt GS, Jayasundaram R, Wareth LA, et al. Thyrotrophin and free thyroxine trimester-specific reference intervals in a mixed ethnic pregnant population in the United Arab Emirates. Clin Chim Acta. 2006;370(1-2):147–151. doi: 10.1016/j.cca.2006.02.008. [DOI] [PubMed] [Google Scholar]
- [52].Hao S, Avraham Y, Bonne O, et al. Separation-induced body weight loss, impairment in alternation behavior, and autonomic tone: effects of tyrosine. Pharmacol Biochem Behav. 2001;68(2):273–281. doi: 10.1016/s0091-3057(00)00448-2. [DOI] [PubMed] [Google Scholar]
- [53].Yang CH, Lee BB, Jung HS, et al. Effect of electroacupuncture on response to immobilization stress. Pharmacol Biochem Behav. 2002;72(4):847–855. doi: 10.1016/s0091-3057(02)00769-4. [DOI] [PubMed] [Google Scholar]
- [54].Huppertz-Kessler CJ, Poeschl J, Hertel R, et al. Effects of a new postnatal stress model on monoaminergic neurotransmitters in rat brains. Brain Dev. 2012;34(4):274–279. doi: 10.1016/j.braindev.2011.07.008. [DOI] [PubMed] [Google Scholar]
- [55].Darre EM, Trier H, Iversen J, et al. Prevention of athletic injuries by changing the regulations. Experiences with a military obstacle track. Ugeskr Laeger. 1991;153(4):281–282. [PubMed] [Google Scholar]


