Abstract
Complexities in the diagnosis of syphilis continue to challenge clinicians. While direct tests (e.g., microscopy or PCR) are helpful in early syphilis, the mainstay of diagnosis remains serologic tests. The traditional algorithm using a nontreponemal test (NTT) followed by a treponemal test (TT) remains the standard in many parts of the world. More recently, the ability to automate the TT has led to the increasingly widespread use of reverse algorithms using treponemal enzyme immunoassays (EIAs). Rapid, point-of-care TTs are in widespread use in developing countries because of low cost, ease of use, and reasonable performance. However, none of the current diagnostic algorithms are able to distinguish current from previously treated infections. In addition, the reversal of traditional syphilis algorithms has led to uncertainty in the clinical management of patients. The interpretation of syphilis tests is further complicated by the lack of a reliable gold standard for syphilis diagnostics, and the newer tests can result in false-positive reactions similar to those seen with older tests. Little progress has been made in the area of serologic diagnostics for congenital syphilis, which requires assessment of maternal treatment and serologic response as well as clinical and laboratory investigation of the neonate for appropriate management. The diagnosis of neurosyphilis continues to require the collection of cerebrospinal fluid for a combination of NTT and TT, and, while newer treponemal EIAs look promising, more studies are needed to confirm their utility. This article reviews current tests and discusses current controversies in syphilis diagnosis, with a focus on serologic tests.
INTRODUCTION
Syphilis, caused by the spirochetal bacterium Treponema pallidum subspecies pallidum, remains a challenging and complex infection to diagnose (1, 2). The inability to readily culture T. pallidum has forced laboratorians to focus on alternate methods for diagnosing syphilis. Microscopic examination of the fluid from ulcerative lesions, from regional lymph nodes, or from the infected tissue has been used since the early 19th century to presumptively diagnose acute cases (1). However, the utility of this test is limited by the inability of even experienced observers to distinguish the organism from other, nonpathogenic treponemes in some specimens (1). While recent advances in molecular methods such as PCR look promising (3), this test largely remains a research tool as it is still not available in many diagnostic laboratories.
Serologic tests for syphilis, with the detection of nontreponemal antibodies (cardiolipin) or antibodies against T. pallidum in all stages of infection, remain the mainstay of diagnosis (1, 2). Nontreponemal tests (NTT) are largely used to monitor the status of infection, while treponemal tests (TT) are primarily used to confirm the presence of treponemal infection. The sensitivity and specificity of both TT and NTT vary with the type of test as well as the stage of syphilis infection. In addition, although T. pallidum subspecies pallidum is the most common species in developed nations, other T. pallidum subspecies exist which differ in their pathogenicity but are >95% homologous by DNA-DNA hybridization (4) and are indistinguishable on serologic testing. This article discusses older tests as well as recent advances in the diagnosis of syphilis with a focus on current testing algorithms for syphilis as well as point-of-care tests (POCT). In addition, current approaches to the diagnosis of congenital and neurosyphilis are discussed.
SEROLOGIC TESTS
Nontreponemal tests.
NTT measure levels of immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies produced by the host in response to lipoidal material (mostly cardiolipin) released from damaged host cells. It also generally believed that some cardiolipin is released by the spirochetes as well (5).
Historically, the antigen was obtained by Wasserman et al. from the liver of an infant that had died of congenital syphilis and was used in an adaptation of an earlier complement fixation test (6). However, it was subsequently noted that the antibodies cross-reacted with other antigens and that an alcohol extract from beef heart was equally suitable for this purpose (1). The identification of the phospholipid cardiolipin as the active antigenic component led to the development of standardized antigens containing cardiolipin, cholesterol, and lecithin (7).
Several NTT have been developed since 1946. The venereal disease research laboratory (VDRL) test (7) is a flocculation test developed using the standardized antigen preparation and remains in use today. The antigen was further modified by the addition of chlorine chloride and EDTA, to produce the unheated-serum reagin test (USR), in which either plasma or unheated serum was an acceptable sample matrix (8).
Later, the rapid plasma reagin (RPR) test was developed. In the RPR test, the antigen suspension incorporates charcoal particles to enhance flocculation (9), while in the toluidine red unheated-serum test (TRUST), the carbon particles were replaced with toluidine red particles (10).
All NTT detect both IgM and IgG antibodies, which are commonly detectable as early as 6 days postinfection (11–13). The sensitivity of NTT during primary syphilis is approximately 75% (14). All NTT in current use are flocculation tests, in which the reaction between the antigen and reagin is evidenced by clumping of particles. Interpretation of flocculation tests is subjective and therefore depends on staff experience, with a minimum of a ±1-dilution margin of error associated with these types of tests.
The NTT, particularly the RPR and VDRL tests, are used worldwide. The RPR test is mainly used to test serum samples, while the VDRL test is now used primarily for testing cerebrospinal fluid (CSF) (1).
NTT are primarily used as qualitative assays for screening in the traditional algorithm or as quantitative assays to help stage infection and to assess the response to treatment. Screening tests are performed using undiluted serum. Results are reported as nonreactive or reactive. Some specimens give a granular or “rough” appearance. A prozone phenomenon occurs in high-titer specimens, mostly with secondary syphilis, which return nonreactive results unless the samples are diluted (15). These cohorts typically remain untreated unless a TT is done irrespective of the RPR/VDRL results, which might be very expensive in resource-limited settings. Most laboratories do not conduct additional testing if the RPR/VDRL test is nonreactive. Reactive specimens are retested in 2-fold dilution series for quantitative results. Titers usually decline significantly after successful therapy and are dependent on the syphilis stage as well as on the presence of HIV coinfection (16–18). Romanowski et al. reported that patients with high pretreatment titers had a greater rate of decline than those with lower titers but were less likely to revert to the negative condition (19). They also reported that patients with repeat infections of early syphilis were less likely to experience seroreversion than those with initial infections (19). Treated patients should be followed for up to 24 months (20); this prolonged follow-up is necessary to ensure that titers decline to the expected extent.
False-positive reactions occur with NTT (21). These are categorized as either acute (occurring for less than 6 months) or chronic. The recognized causes of acute false-positive reactions include other febrile illnesses, immunizations, and pregnancy (22). Patients with acute false-positive reactions should be retested in 3 to 6 months. Chronic false-positive reactions are associated with hepatitis C virus infection (23), connective tissue diseases, intravenous drug use, malignancy, older age, malaria, Chagas disease, tuberculosis, and leprosy (1).
NTT, particularly the RPR and VDRL tests, are cheap and simple to perform but are manual in nature; there is currently no appetite for commercial enterprises to automate these tests.
Treponemal tests.
The TT were developed using the Nichols strain of T. pallidum (1, 24) and utilizing either whole cells or antigens derived from cells of T. pallidum. TT assays can detect either IgM or IgG antibodies, depending upon the specific assay kit. Most diagnostic laboratories utilize the T. pallidum hemagglutination assay (TPHA), the T. pallidum particle agglutination assay (TPPA), and/or the fluorescent treponemal antibody absorption assay (FTA-ABS) (25, 26). More recently, Western blotting (WB) and the line immunoassay (LIA) have been added to the portfolio of treponemal tests. Antibodies detected by treponemal assays arise earlier than those detected by NTT and typically remain detectable for life, even after successful treatment.
The TPHA and TPPA are indirect agglutination tests in which surface antigens extracted from T. pallidum cells are coated onto red cells (TPHA) or gelatin particles (TPPA) and mixed with the test serum. Serum containing specific T. pallidum antibodies reacts with the antigen-sensitized red cells or gel particles, causing agglutination. However, the sensitivity and specificity of both tests are not optimal for primary cases. The TPPA has been found to be superior to TPHA, possibly due to the homogenous structure of gel particles (27).
The FTA-ABS is an indirect immunofluorescent staining assay in which fixed cells of the Nichols strain of T. pallidum are exposed to the test serum after the serum has been absorbed with a sorbent; the sorbent is an extract from a nonpathogenic T. phagedenis strain (sometimes referred to as the Reiter strain). After being washed to remove unbound antibody, the reaction mixtures are incubated with fluorescein-conjugated anti-human globulin. In a positive test, the presence of antibodies to T. pallidum in the serum specimen is indicated by the appearance of fluorescent spirochetes by examination with a fluorescence microscope. The FTA-ABS is slightly more sensitive than either the TPHA or the TPPA and is usually the first serologic test to become reactive, during the primary stage of the disease (1). However, the reading of the FTA-ABS is subjective and occasionally gives false-positive (nonspecific fluorescence) results.
Both IgM WB and IgG WB utilize antigens of T pallidum which are fractionated in the presence of SDS by polyacrylamide gel electrophoresis. The resolved protein bands are then transferred by electrophoresis to a nitrocellulose membrane, dried, and cut into pieces, with one strip used per patient sample. The patient serum is diluted and incubated with individual T. pallidum antigen strips. If T. pallidum antibodies are present in the serum, then they bind to T. pallidum proteins. Results showing reactivity to T. pallidum species-specific bands are considered positive or reactive. The LIA is an alternate to WB, but this technique utilizes purified antigens as well as synthetic peptides and involves coating of the sample onto a nylon strip with plastic backing in the form of discrete lines. This is easier to read than whole-cell-based Western blot strips. The strips are available in IgM and IgG formats, but the majority of clinical laboratories utilize IgG LIA strips containing TpN 47, TpN 17, TpN 15, and TmpA protein antigens. In one study, sensitivity ranged from 98.5% to 99.9% and specificity ranged from 98.1% to 99.9% (28). A potential application of these assays is to reconcile discordant results, since they allow the users to visualize the reacting antigens (29–31).
In recent years, newer treponemal tests have been developed which utilize recombinant antigens derived from T. pallidum. This approach may allow greater specificity and sensitivity and also promote standardization. Recombinant treponemal assays suitable for screening are available in enzyme-linked immunosorbent assay (ELISA) format (for IgG, for IgM, and for total immunoglobulins), in chemiluminescence immunoassay (CIA) format, and in multiplex bead enzyme immunoassay (EIA) format. The chemiluminescence and multiplex bead immunoassays are capable of high throughput and even random-access testing, but each is capable of running on the manufacturer's platform only. Limited data suggest that the performances of these assays for screening differ between testing platforms (32–34); however, reproducibility of results may be better achieved by the CIA (35).
Accumulating experience with treponemal assays as screening tests suggests that these assays generate a small proportion of false-positive results which cannot be explained by a history of previous infections. Since these tests have not been previously been applied as tests for screening populations, it is not surprising that apparently false-positive results occur in a small proportion of the population (36). Similarly to older treponemal assays, these tests can give false-positive results with other spirochetal infections, such as borrelial infections, as well as from commensal microorganisms (37). Further studies will be necessary to quantify the rate of false-positive results, although, considering their higher analytical sensitivity and also the absence of a gold standard, it is difficult to prove that positive results obtained using these platforms are necessarily false positives, hence demanding a thorough search into the patient's past medical and social history.
DIAGNOSTIC ALGORITHMS
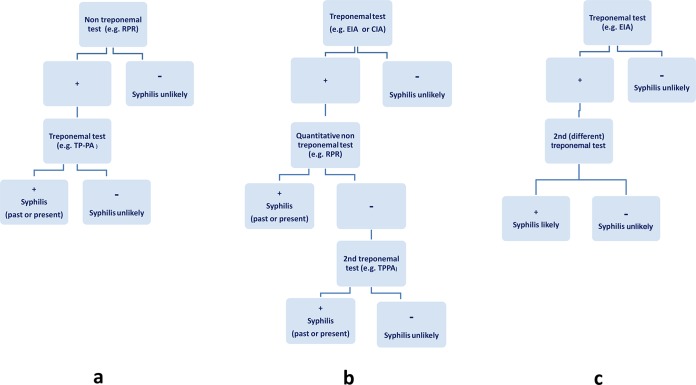
There are currently two commonly used approaches to the serological diagnosis of syphilis: the traditional algorithm (Fig. 1a) and a second algorithm—commonly referred to as a reverse-sequence algorithm (Fig. 1b). A variation of the reverse-sequence algorithm is recommended by the European Centre for Disease Prevention and Control (ECDC) (Fig. 1c). The latter version of the reverse-sequence algorithm is not currently in use in North America.
FIG 1.
Testing algorithms for syphilis diagnosis. (a) Traditional algorithm. (b) Reverse algorithm. (c) European Centre for Disease Prevention and Control (ECDC) reverse algorithm. +, positive result; −, negative result; RPR, rapid plasma reagin; TP-PA or TPPA, Treponema pallidum particle agglutination test; EIA, enzyme immunoassay; CIA, chemiluminescence immunoassay (36, 39, 48).
Traditional algorithm.
Traditionally, syphilis serologic testing has been performed using a NTT such as the RPR or VDRL test, with positive results then confirmed using a specific TT such as TPPA or FTA-ABS (Fig. 1a). This algorithm is currently endorsed by the U.S. Centers for Disease Control and Prevention (CDC) (20).
The RPR test is relatively cheap and can be performed in nearly any laboratory setting. However, traditional RPR-based screening may not always be followed by a treponemal test, especially in resource-limited settings, and may therefore miss some previously treated, early untreated, and late latent cases where the RPR test is nonreactive. In 1982, a scientific group convened by the World Health Organization recommended that both a NTT and a TT be used for screening and diagnostic purposes but acknowledged that a NTT alone could be used for screening in areas of high syphilis prevalence and limited resources (38). This algorithm is cost-effective for small laboratories with a low volume of specimens but has significant limitations, including the use of a screening test that lacks specificity, requires manual operation, and is subjective (39). Some laboratories prefer to use the VDRL test as the screening test; however, poorer sensitivity, especially in cases of primary syphilis, may be an issue (1).
Reverse algorithm.
In recent years, due to the need for efficiencies in high-volume screening as well as the need to address the ergonomic stress of pipetting large numbers of samples, many laboratories have changed their diagnostic approach and now screen using automated or semiautomated treponemal antibody assays in which the blood sample is tested using an EIA. Positive samples are then tested with a quantitative NTT (e.g., an RPR or VDRL test). If the test results disagree, the specimen is then tested using a second treponemal test (Fig. 1b). The TPPA is generally recommended for use as the confirmatory test based on published data suggesting the enhanced sensitivity and specificity of this assay (40). Other data, however, indicate comparable levels of performance of several treponemal tests (32). This algorithm is currently endorsed by the Association of Public Health Laboratories, the United Kingdom Health Protection Agency, and the International Union against Sexually Transmitted Infections (41–43).
All treponemal EIA kits utilize 15.5-kDa, 17-kDa, 45.5-kDa, and 47-kDa treponemal proteins either singly or in combination. Some experts recommend using second- or third-generation treponemal tests which contain newer and more-specific antigens, but these tests are not yet commercially available (44). Based on local laboratory performance data, some laboratories have elected to use an RPR cutoff value above which further testing is discontinued; for example, in Quebec, Canada, specimens testing EIA positive with RPR values of ≥1:8 dilutions almost always have the positive result confirmed when tested with a second TT, and a second TT is not routinely done in such situations (Bouchra Serhir, personal communication). Park and colleagues have also suggested the possible utility of the quantitative optical density index (ODI) value; they showed that a mean ODI value of ≥12.0, as well as a higher range of ODI values, was seen in TPPA-positive individuals (45).
The interpretation of results from and optimal management of patients testing positive with a screening EIA but with a nonreactive RPR result are unclear. A Centers for Disease Control and Prevention (CDC) evaluation of treponemal screening tests among four New York City laboratories showed that 56% of persons with a positive treponemal EIA result had negative results by a nontreponemal test and that, when a second, different treponemal test was used, 83% of patients tested positive (46). They encouraged clinicians to consider providing treatment for late latent syphilis in such cases (EIA positive, RPR nonreactive, second TT reactive), unless the history or results of a physical examination suggested a recent infection, as treatment might reduce the chance of progression to tertiary complications (46). Park et al. also concluded in their analysis that the use of a second TT is useful to guide clinical management, especially in low-prevalence settings (45). It was acknowledged, however, that overtreatment may occur, as the patients may not recall previous diagnosis or treatment, infections may have resolved without treatment, or patients may have received an antibiotic with activity against T. pallidum for treatment of other infections (45, 46).
If the second TT result is positive, this is generally considered to confirm a diagnosis of syphilis, but if the second TT result is negative, a third TT may be helpful and is generally recommended as a “tie-breaker” (2). At M. G. Morshed's laboratory (British Columbia Public Health Microbiology and Reference Laboratory), a positive result by the chemiluminescent microparticle immunoassay (CMIA) (Siemens) is followed by a NTT as well as a second TT (TPPA). If the TPPA result is low positive (1+), the sample is tested using a third TT (Inno LIA). In M. G. Morshed's experience, false positivity is greatly reduced by conducting a third TT but clinical correlation remains challenging in some situations, e.g., in cases of low (1.1 to <10)-index-titer CMIA samples, nonreactive RPR, nonreactive and equivocal TPPA, or low-positive (1+) Inno LIA results. If early infection is suspected, then the tests should be repeated 2 to 4 weeks later.
Reverse-screening algorithms allow automation and increased sample throughput but also address ergonomic issues for technologists and detect more cases of early as well as latent syphilis (35, 47, 48). These algorithms, employing fully automated systems, have been adopted by many private as well as public laboratories in Europe and North America.
This approach also has limitations, with an increase in the potential for false positives (36, 46). Furthermore, initial setup costs as well as ongoing laboratory operational costs may be also be higher (49, 50).
Recently, the European Centre for Disease Prevention and Control (ECDC) modified the CDC reverse algorithm (Fig. 1c): a reactive treponemal screening test is followed by a second (and different) TT but is not accompanied by a NTT (42).
Comparison of the three algorithms.
Ideally, a screening test should be simple and easy to use, provide rapid results to enhance faster therapeutic interventions, and have the sensitivity, specificity, and positive and negative predictive values suitable for use in both low- and high-prevalence populations. It also needs to be cost-effective. Meeting all these testing criteria is not simple, and each of the previously described testing algorithms has its advantages and limitations.
In a cross-sectional study of 24,124 subjects by Tong et al., every serum sample was simultaneously tested using the RPR test, TPPA, and CIA, and using the results of clinical diagnoses of syphilis as the gold standard, the researchers evaluated the diagnostic accuracy of each of the 3 algorithms (48). They reported that the traditional algorithm had a missed-diagnosis rate of 24.2% and had only 75.81% sensitivity (48). Both the reverse and ECDC algorithms had higher diagnostic efficacy than the traditional algorithm, with sensitivity of 99.38% to 99.85%, specificity of 99.98% to 100%, and accuracy of 99.93% to 99.96%. While their study supported the use of the ECDC algorithm, they acknowledged that a nontreponemal assay is recommended for determining serological activity and the effect of syphilis treatment.
Owusu-Edusei conducted a cohort decision analysis to estimate the expected costs and effects of the traditional and reverse algorithms in a low-prevalence setting such as would be found in the United States (50). They demonstrated that, while the reverse algorithm resulted in the treatment of 99% of syphilis cases, it was more expensive overall because it resulted in a significantly higher number of follow-ups (three times as many) and overtreatment. Another study by the same authors examined the health and economic outcomes of the screening algorithms in low- and high-prevalence settings (51). The 2-step algorithms detected and treated the same number of individuals, but the traditional algorithm was more cost-effective in a low-prevalence setting ($1,400 versus $1,500 per adverse outcome prevented) and cost less ($102,000 versus $84,000) in a high-prevalence setting (51).
Mishra et al. conducted a retrospective study of the impact of reverse screening in the laboratory diagnosis of syphilis and reported that reverse screening identified a higher percentage of screen-reactive patients (2.24% versus 0.59%) than the traditional algorithm (47). Although the authors indicated that this may represent the identification of a large number of individuals who had received syphilis treatment in the past, it may also have improved the detection of late latent and early primary syphilis. Another recent Canadian study reported an increase in the number of late latent cases of syphilis which required additional public health follow-up (52). The use of treponemal tests for screening, followed by nontreponemal tests, also resulted in higher overall testing costs; this was, largely, due to the substantial increase in the number and cost of confirmatory tests (51).
Given the pros and cons of each diagnostic algorithm, the decision to use a treponemal or nontreponemal assay as the first screening test should be based on a combination of factors: local syphilis prevalence, the expected workload (laboratory and clinical), the requirement for automation, and the available budget for labor and consumables (49).
Regardless of which algorithm is used, it is important that the clinician always take other factors such as sexual behavior, medical history, previous treatment history of syphilis, etc., into consideration, and if the clinical assessment suggests possible or probable syphilis and the syphilis screening test is nonreactive, the laboratory test should be repeated after 2 to 4 weeks. If the test is still nonreactive, early syphilis is unlikely.
POCT
Syphilis point-of-care tests (POCT) are widely available for use in developing countries, where they expand the range of settings in which sexually transmitted infection (STI) testing can be undertaken, thus facilitating earlier diagnosis and access to rapid treatment and support (53, 54).
With the global resurgence of syphilis in many developed nations, including among members of harder-to-reach populations such as men who have sex with men (MSM) and sex trade workers (55–57), POCT also offer the unique ability to offer immediate testing and treatment in a single encounter to mitigate further transmission of syphilis, making this an attractive alternative to standard testing (53, 58).
Currently, there are of 2 varieties of syphilis POCT in use: (i) immunochromatographic strip (ICS) tests, which work by having a test strip with a line that is impregnated with treponemal antigens that react with antibodies to syphilis in whole blood or serum to produce a visible result on the test strip, and (ii) particle agglutination tests (PATs), which use gelatin particles coated with treponemal antigens that clump together on a test tray when combined with whole blood or serum containing antibodies to syphilis. Most POCT are ICS-based tests (59).
A systematic review of 15 studies using syphilis POCT conducted at antenatal or STI clinics reported median sensitivity of 0.86 (interquartile range [IQR], 0.75 to 0.94) and median specificity of 0.99 (IQR, 0.98 to 0.99) (54). The researchers also reported good positive predictive values over a range of syphilis prevalences. A recent meta-analysis summarized the available performance data from 18 syphilis POCT, most of which were ICS-based tests (60). The meta-analysis found that the Determine rapid test (Abbott Diagnostics, United Kingdom) using a serum sample had the best sensitivity (92.03%; 95% confidence interval [CI], 87.2% to 95.8%) and that Syphicheck (Qualpro, India) had the best specificity (99.4%; 95% CI, 98.9% to 99.8%). An Australian laboratory-based study of four syphilis POCT using stored sera reported that the Determine test had the highest overall sensitivity, with significantly higher test sensitivities among high-RPR-titer (RPR ≥ 1:8) tests (61). POCT results were compared to treponemal immunoassay reference test results.
Only one test, the Syphilis Health Check (Trinity Biotech, Jamestown, NY, USA), is Food and Drug Administration (FDA) approved for use in the United States (62). Limited data are available to confirm whether the sensitivity of the syphilis POCT is maintained in HIV-infected individuals (63) and in those with high RPR titers (63, 64).
Available data on the antenatal cost-effectiveness of POCT show that the ICS TT tests are cost-effective for the detection of maternal syphilis in low-resource settings compared to either standard 2-test algorithms (i.e., NTT followed by TT) or a NTT alone (65–67). Owusu-Edesei and colleagues recently reported that a screening strategy employing an ICS TT cost less than a dual-POCT (TT and NTT) strategy in a high-prevalence setting but that the dual-POCT strategy may significantly reduce overtreatment (68). No cost-effectiveness data are available for developed countries.
Because a positive treponemal POCT result may indicate new or old infections, a quantitative nontreponemal test is often helpful. However, there are no commercially available nontreponemal POCT available as a single test at this point. Two commercially available dual tests are currently available. Castro et al. evaluated a novel POCT (Chembio Diagnostics System Inc., Medford, NY, USA) for the simultaneous detection of nontreponemal and treponemal antibodies in sera (69). The reactive concordance of the nontreponemal result compared to the RPR test result was 98.4% when the RPR value was ≥1:2, but when the RPR value was ≤1:1, the sensitivity declined to 88%. Compared to the TPPA, the reactive and nonreactive concordances of the treponemal line were 96.5% and 95.5%. A recent study from China confirmed good sensitivity and specificity of this test for both treponemal and nontreponemal antibodies in serum, plasma, and whole blood (70). Span Diagnostics (Gujarat, India) also makes a dual test (http://www.span.co.in/#), but no published data on its performance in the field are available.
The choice of test kit and specimen type is important when deciding which kit will perform optimally in any given field setting. For example, Campos et al. (71) reported lower sensitivities with whole-blood (finger prick) specimens which may have been due to inadequate lighting, lack of use of heparinized capillary tubes for collection of whole blood, false negatives due to previously treated syphilis, and a low proportion of samples reactive at low titers.
Because POCT are often performed by inexperienced non-laboratory workers outside a laboratory, results can be variable. Herring et al. (72) reported variability with respect to test lots and day-to-day testing and differences between testers. Judgment may be based on interpretation of a band being positive or negative in an ICS test or of agglutination strength in a PAT. For these reasons, it is generally recommended that procedural manuals be developed in conjunction with a local reference laboratory to include a control and proficiency-testing program and that quality assurance (QA) programs be developed.
It should be noted that, similarly to other screening tests for syphilis, a single POCT for syphilis may not be adequate for the diagnosis of syphilis and should follow the recommended testing algorithms as described above.
NEUROSYPHILIS
“Neuroinvasion,” or spread by T. pallidum to the CSF and meninges, can occur early in infection, even before the clinical manifestations of primary syphilis occur (73). In most cases, the organisms are cleared spontaneously, but in others, symptomatic disease can occur (74). There are no gold standard tests for the diagnosis of neurosyphilis, but definitive diagnosis usually requires serologic confirmation of syphilis infection (any stage) together with a reactive cerebrospinal fluid VDRL test (74). In addition, the criteria for asymptomatic neurosyphilis have not been standardized and have been applied inconsistently in research studies, further complicating the interpretation of tests used to diagnose neurosyphilis (75, 76).
Most experts and guidelines no longer recommend routine lumbar puncture (LP) in all patients with syphilis, but all agree that it is indicated in patients with neurological symptoms or signs suggestive of neurosyphilis, including ocular or ophthalmic involvement (20, 74, 77). It should be noted, however, that the results of a CSF examination are frequently normal in patients with auditory involvement (78). Whenever the eye or ear or both are involved, the patient case is managed as a neurosyphilis infection regardless of CSF findings (76). The value of identifying asymptomatic neurosyphilis, particularly in HIV-coinfected patients, remains controversial (74, 77). It is generally accepted that HIV-coinfected persons are more likely to develop neurosyphilis and that treatment of asymptomatic neurosyphilis prevents the development of symptomatic neurosyphilis (74). Current CDC guidelines recommend LP in cases of (i) patients who fail to achieve an adequate serologic decline in serum NTT titer (defined as failure of the nontreponemal test titer to decline 4-fold in the 12 to 24 months following treatment), (ii) active tertiary syphilis (e.g., gumma and aortitis), and (iii) congenital syphilis (20). Several studies have reported that HIV-coinfected patients with clinical and CSF abnormalities consistent with neurosyphilis have a CD4 count of ≤350 cells/ml and/or an RPR titer of ≥1:32 (79–81). However, current CDC guidelines do not recommend routine LP in these settings since management based on CSF examination in this setting has not been associated with improved clinical outcomes (20). Some experts, however, continue to recommend LP in all HIV-coinfected patients and in those with an RPR level of greater than 1:32 dilutions (74).
The tests available for syphilis detection in CSF can be divided into direct detection and antibody assay methods. CSF T. pallidum PCR methods for the molecular detection of treponemes in CSF were published by Hay et al. in 1990 (82). Subsequent studies have shown that this assay is not as sensitive as the rabbit infectivity assay, which can detect as few as 1 to 2 viable treponemes whereas at least 10 organisms are needed for PCR positivity (83). Although available, this assay is generally restricted to reference facilities. At this stage, PCR is not a sufficiently sensitive tool for the routine detection of T. pallidum in serum or CSF. When a CSF sample tests positive for T. pallidum in a well-validated assay, this result is definitive for the presence of the organism.
Antibody assays include both NTT and TT. The CSF VDRL test remains the standard for the diagnosis of neurosyphilis today, but although it has very high (99.8%) specificity, its sensitivity is only 50% (range, 30% to 70%) (84). Consequently, while a negative CSF VDRL test result does not exclude the possible presence of neurosyphilis, a positive finding is strongly suggestive of the disease. Rare false-positive VDRL results do occur, and additional testing, such as by FTA-ABS, can represent an alternative verification assay, together with serologic verification of syphilis infection. Patients who are serologically negative for syphilis but have a positive CSF VDRL test result are unlikely to have neurosyphilis. An additional advantage of this assay is that it is quantitative, and changing titers can be used to monitor the effectiveness of treatment. The RPR test is easier to perform in CSF samples, but a recent study reported that it too resulted in a high false-negative rate and that insufficient data exist at present to replace the VDRL test with the RPR test in CSF analyses (85).
Earlier data suggested that the CSF FTA-ABS is very sensitive but not specific and that a negative test result may help to rule out neurosyphilis (86). A recent systematic review, however, reported that the negative predictive value was dependent on the specificity of the test and the prevalence (i.e., pretest probability) of neurosyphilis: the higher the prevalence, the lower the negative predictive value (87). The authors concluded that a negative CSF result from a treponeme-specific antibody test may not exclude a diagnosis of neurosyphilis when the clinical suspicion for neurosyphilis is high (87). In addition, due to the high sensitivity of the FTA-ABS with respect to detecting very low levels of contaminating serum antibody, it is particularly important to prevent contamination of the CSF specimen with serum or plasma since small amounts of blood contamination of the CSF may give false-positive test results with the FTA-ABS (88). Despite effective treatment, the CSF FTA-ABS results can continue to be positive for an extended period. In serial follow-up testing of treated patients, CSF FTA-ABS is not useful (89).
Some clinicians have proposed the use of the Treponema pallidum particle agglutination assay (TPPA) as an alternative to the FTA-ABS (90, 91). In two studies, this CSF assay performed similarly to the CSF FTA-ABS, with both assays identifying true cases of neurosyphilis, based upon a reactive VDRL result. However, both also identified antibody in some control patients with no evidence of neurosyphilis. The TPPA does not seem to have found general use in many clinical laboratories, as part of the protocol requires calculation of a serum or CSF ratio or index (92), which may not always be possible if a matched blood sample was not drawn at that time of the lumbar puncture. Although a positive CSF-VDRL test result is diagnostic, this test is relatively insensitive, and most clinicians rely on indirect markers of central nervous system (CNS) inflammation, such as an elevated white blood cell (WBC) count or protein in the CSF (93). Patients who are HIV infected and have low CD4 counts may present with serologic and CSF findings that are different from those of immunocompetent hosts (94). Significant differences, including a higher cell count, higher protein levels, and lower glucose levels in the HIV-infected group, have been noted in CSF measurements in comparisons of HIV-positive patients with HIV-negative patients with syphilis; these changes have been attributed to the presence of HIV itself (95).
It is generally accepted that patients with a reactive CSF-VDRL test result should be treated for neurosyphilis (20). As well, those patients with a CSF white cell count of 5 × 10E6/liter and greater should also be treated due to the poor sensitivity of the CSF-VDRL test and the association between abnormal CSF cell counts and neurosyphilis. Current CDC guidelines (20) also suggest that patients with a high level (>45 mg/dl) of CSF protein should have their CSF tested by the FTA-ABS, as it is more sensitive that the CSF-VDRL test. If the FTA-ABS gives a positive result, this is highly suggestive evidence for neurosyphilis, especially if the patient has features compatible with neurosyphilis, which is often a difficult diagnosis to make given the protean nature of this disease. HIV-coinfected patients with a reactive CSF-VDRL result, as well as those with a CSF white cell count of 20 × 10E6/liter and greater, should be treated for neurosyphilis using the recommended treatment regimens (20); using a higher cutoff value (>20 WBC/mm3) might improve the specificity of neurosyphilis diagnosis (79).
Follow-up of patients with neurosyphilis typically involves clinical monitoring as well as regular follow-up of serum RPR levels (20). In addition, those with abnormal CSF findings should have follow-up CSF at 6-month intervals until normalization of CSF parameters (20).
In adults, CSF pleocytosis is generally the first measure of improvement and a decline should occur over about 6 months. The CSF-VDRL titer should decline (4-fold within a year) if it is initially high, but it may take years to revert to negative (96). A persistent, low CSF-VDRL titer after a course of treatment may warrant retreatment, but if CSF pleocytosis and elevated protein levels have resolved and the serum VDRL titer has not risen, additional treatment is unlikely to be beneficial (97). Elevated protein levels, if present, begin to decline during the first 6 months but can take up to 2 years to return to normal (98). CSF protein levels may decline more slowly in patients who are neurologically abnormal than in those who are neurologically normal (99). A normalization of serum RPR levels is predictive of normalization of CSF and clinical abnormalities after treatment of neurosyphilis (100).
All CSF laboratory parameters normalize more slowly in patients infected with HIV (99). The possibility of treatment failure should be considered if there is clinical progression or an increase in RPR/VDRL levels by ≥2 dilutions at any time or if CSF pleocytosis fails to resolve 2 years posttherapy.
PRENATAL SYPHILIS SCREENING AND CONGENITAL SYPHILIS
Classically, congenital syphilis is divided into two clinical syndromes: early (diagnosis during the first 2 years of life, including stillbirths) and late (diagnosis after 2 years of life, with mainly tooth, bone, and central nervous system manifestations).
Effective prevention and identification of congenital syphilis depend primarily on the identification of syphilis in pregnant women and therefore on the routine screening of all pregnant women for syphilis. A systematic review reported that antenatal syphilis interventions, including screening, could reduce perinatal stillbirth and death rates by 50% (101). In addition, antenatal screening for syphilis has been shown to be cost-beneficial even in developed countries with a relatively low prevalence of syphilis (102). Initial screening should ideally be performed in the first trimester and should be repeated at 28 weeks and again at delivery in women at high risk of acquiring syphilis (20). More-frequent screening may be indicated in women at particularly high risk for acquisition (or reinfection) with syphilis in pregnancy (e.g., sex trade workers) (103). In addition, consideration should be given to rescreening all pregnant women in areas experiencing heterosexual outbreaks of syphilis, regardless of the woman's risk profile; this is especially important in areas where congenital syphilis cases have been reported in women with no personal risk factors for syphilis (103). Screening in the first trimester and at 28 to 32 weeks is intended to prevent the transmission of syphilis to the fetus by maternal treatment in pregnancy, while screening near term or at delivery serves primarily to detect congenital cases and allow for early treatment.
Any woman delivering a hydropic or stillborn infant at ≥20 weeks gestation should also be screened for syphilis. No newborn should be discharged from a hospital prior to confirmation that either the mother or newborn infant has had syphilis serology undertaken during pregnancy or at the time of labor or delivery and that the results will be followed up (20).
Screening of the mother's serum (rather than testing cord blood or the infant's serum) is preferred because of the ease of obtaining good-quality blood samples and the ability to provide maternal disease staging. Moreover, serologic tests performed on infant serum can be nonreactive if the mother's nontreponemal serologic test result is of low titer or the mother was infected in late pregnancy (20). There have been case reports of “missed” congenital syphilis with a negative maternal screen at delivery that likely represented mothers with early acute syphilis infection and nonreactive nontreponemal screening tests at the time of delivery (104, 105).
Pregnancy may cause false-positive treponemal as well as nontreponemal tests; therefore, similarly to management of nonpregnant patients, it is important that confirmatory tests be conducted before making a diagnosis of syphilis (1, 2). Seropositive pregnant women should be considered infected unless an adequate treatment history is clearly documented and sequential serologic antibody titers have declined.
The assessment and management of infants with reactive syphilis serology or born to mothers with reactive syphilis serology are complex. The stage of maternal syphilis, gestational age of the fetus at the time of acquisition and treatment of infection, adequacy and timing of maternal treatment, and immunological response of the fetus can cause varied manifestations of congenital syphilis. The diagnosis of congenital syphilis is based on a combination of clinical and laboratory evaluations (106). Diagnosis is further complicated because up to 60% of infected infants are asymptomatic at birth or have subtle, nonspecific findings (107, 108). Common early signs include hepatosplenomegaly, rash, fever, neurosyphilis, pneumonitis, and snuffles, while common laboratory abnormalities include Coomb's negative hemolytic anemia (58%) and elevated levels of transaminases and alkaline phosphatase in blood (106). Results of radiographs of long bones may also be abnormal.
Serologic tests for syphilis remain the mainstay of diagnostic tests for congenital syphilis. For serological tests in the newborn, venous blood should be used in preference to cord blood as the latter is frequently contaminated with maternal blood (109). Parallel testing of both the mother's serum and the infant's serum at delivery with the same nontreponemal test and, preferably, by the same laboratory will help to determine the significance of the serologic findings in the infant. A titer in the infant's serum that is higher than the mother's titer by 4-fold or greater at delivery is strongly suggestive of congenital infection (107). However, the absence of an infant's titer that is higher than the mother's titer by 4-fold or greater does not exclude the possibility of congenital infection as studies of serum pairs from infected mothers and infants show that fewer than 30% of infants have higher titers than their mothers (110). IgM antibodies can be detected in more than 80% of symptomatic infants, but data on the sensitivity of antibody assays in asymptomatic infants are limited (111, 112). Moreover, the traditional FTA-ABS IgM test is technically difficult to perform and steps to remove IgG and interference by the presence of the rheumatoid factor (19S FTA-ABS IgM test) have resulted in increased specificity but loss of sensitivity with this assay (113). One assay performed using the IgM capture format and labeled treponemal antigens was more sensitive than the 19S FTA-ABS IgM test (111), but the number of patients included in the study was small. Another study reported an immunoblot assay for IgM which was more sensitive and specific than the 19S FTA-ABS IgM test (114), but it was an in-house assay that required special reagents and expertise. Due to the poor performance of currently available IgM tests for syphilis, including a commercially available immunoglobulin (IgM) test approved for use by the U.S. FDA (Captia Syphilis-M EIA; Trinity BioTech, Bray, Ireland), they are not currently recommended by the CDC (20).
NTT titers in infants should decline by age 3 months and should be nonreactive by age 6 months in cases in which the infant was not infected (i.e., if the reactive test result was due to passively transferred antibodies) or was infected but adequately treated (20). The serologic response to treatment is expected to be slower for infants treated after the neonatal period. Stable or rising titers might indicate persistent infection and are an indication for repeat evaluation and treatment. The diagnosis of congenital syphilis can be excluded if the NTT becomes nonreactive before the age of 6 months in an infant who has not received treatment (115). Similarly, a negative TT result can be used to exclude the possibility of congenital syphilis if the tests are nonreactive before the age of 1 year in an infant who has not received treatment.
TT results can remain positive despite effective treatment. Passively transferred antibodies can persist in an infant up to age 15 months, and, as such, a reactive TT after age 18 months is diagnostic of congenital syphilis; such infants require full evaluation and treatment for congenital syphilis if treatment was not provided or if treatment can be deemed to have been inadequate by a review of the treatment history (116). A study using the FTA-ABS test showed that only about half of the group of infants with clinical or laboratory evidence of congenital syphilis at birth had reactive FTA-ABS results at 12 months of age (110). A recent Canadian case series of infants with congenital syphilis reported that 69% of infants showed seroreversion in their treponemal tests by 18 months and that infants who did not show seroreversion in their TT were statistically more likely to have had delayed treatment and to have had higher maternal RPR titers at birth (117).
Given the similar modes of transmission of syphilis and HIV, all infants with reactive syphilis tests should also undergo concurrent HIV testing (118). Concurrent syphilis infection has been shown to be associated with vertical transmission of HIV (119).
CONCLUSIONS
An accurate and simple approach to the diagnosis of syphilis remains elusive, and diagnosis continues to require a comprehensive assessment of the patient, including risk exposure, the presence of compatible clinical symptoms and signs, and laboratory tests. While direct tests (such as microscopy or PCR) are helpful in early infection, serologic tests remain the mainstay of syphilis diagnosis.
Traditional syphilis algorithms which commence testing with a NTT have been in place for decades and remain in wide use globally. A number of rapid, cheap, simple, and accurate treponemal point-of-care tests are now available worldwide and will have optimum benefit in areas with high syphilis prevalence and in members of hard-to-reach populations, who are less likely to return for follow-up. Congenital syphilis continues to remain a scourge worldwide, and the priority for prevention remains universal screening of all pregnant women. Due to limited advances in the serologic diagnosis of congenital syphilis, management still requires an assessment of maternal treatment and serologic response as well as clinical and laboratory investigation of the neonate. Diagnosis of neurosyphilis continues to be challenging and requires the collection of cerebrospinal fluid for a combination of NTT and TT as well as other CSF parameters.
In recent years, newer syphilis testing algorithms that reverse the order of testing have used automated assays that detect treponemal antibodies and have gained in popularity as they are easily performed on high-volume specimens, thus reducing labor costs. They have the added benefit of being at least as sensitive as the nontreponemal screening tests. As shown by reflex testing of positive immunoassay specimens with a quantitative RPR test and a second TT, the reverse algorithm is nearly 100% specific. However, none of the current diagnostic algorithms are able to distinguish current from previously treated infections. The decision to use the traditional or reverse algorithm should be made based on a combination of the local syphilis prevalence, the expected (clinical and laboratory) workload, the requirement for automation, and the available budget. Since it is anticipated that more laboratories will continue to use or switch to newer algorithms for syphilis testing, laboratorians and clinicians will have to become more comfortable with interpreting test results when testing starts with a treponemal EIA. Further research is needed to evaluate the utility of optical density index (ODI) values for the newer EIAs and/or RPR cutoffs to guide the need for additional testing in reverse algorithms; if a second or third TT were to be omitted from the testing algorithm, costs would be significantly reduced. Additional performance and cost-effectiveness evaluations of dual POCT in a variety of low- and high-prevalence settings is also much needed. It is hoped that additional clinical and laboratory experience with patients diagnosed and managed with the newer algorithms and tests will ultimately contribute to the goal of improving syphilis prevention and control.
ACKNOWLEDGMENT
Thanks are due to Min-Kuang Lee for his technical assistance.
Biographies

Muhammad G. Morshed received his Ph.D. from the Yamaguchi University School of Medicine, Japan, in 1994. He completed his postdoctoral work in the same university as well and also at the Research Institute of Tuberculosis in Tokyo, Japan. He is currently a Clinical Microbiologist and Program Head of Zoonotic Diseases and Emerging Pathogens at the BC Public Health Microbiology and Reference Laboratory, Provincial Health Services Authority Laboratories. He has also been a Clinical Professor in the Department of Pathology and Laboratory Medicine, Faculty of Medicine, University of British Columbia, since 1997. He also worked at the International Centre for Diarrhoeal Disease Research, Bangladesh, Bangladesh Institute of Child Health, and Jahangirnagar University Bangladesh from 1982 to 1996. His laboratory is responsible for specialized serology testing, molecular testing, and microbial fingerprinting, program evaluation, and research on zoonotic and emerging pathogens, with particular interest in spirochetes, namely, Treponema pallidum, Borrelia burgdorferi, and Helicobacter pylori.

Ameeta E. Singh completed her undergraduate medical degree at Nottingham University in the United Kingdom in 1989. This was followed by an internal medicine residency and infectious diseases fellowship at the University of Alberta (Edmonton, Canada), which were completed in 1996. In 2002, she completed a Master of Science program in Epidemiology at Harvard University (Boston, MA, USA). She was the provincial consultant for sexually transmitted and blood-borne infections (STBBI) in Alberta (Canada) for 10 years and the Medical Director of the Edmonton STI Clinic from 1998 to 2014. She is currently a Clinical Professor with the University of Alberta's Division of Infectious Diseases. Although she maintains a general infectious diseases and HIV/STI practice, her clinical and research interests include STBBI, in particular, syphilis.
REFERENCES
- 1.Larsen SA, Steiner BM, Rudolph AH. 1995. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev 8:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seña AC, White BL, Sparling PF. 2010. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis 51:700–708. doi: 10.1086/655832. [DOI] [PubMed] [Google Scholar]
- 3.Gayet-Ageron A, Lauterschlager S, Ninet B, Perneger TV, Combescure C. 2013. Sensitivity, specificity, and likelihood ratios of PCR in the diagnosis of syphilis: a systematic review and meta-analysis. Sex Transm Infect 89:251–256. doi: 10.1136/sextrans-2012-050622. [DOI] [PubMed] [Google Scholar]
- 4.Norris SJ. 1993. Polypeptides of Treponema pallidum: progress toward understanding their structural, functional, and immunologic roles. Treponema pallidum Polypeptide Research Group. Microbiol Rev 57:750–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pope V, Norris SJ, Johnson RE. 2007. Treponema and other human host-associated spirochetes, p 987–1003. In Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA (ed), Manual of clinical microbiology, 9th ed, vol 1 ASM Press, Washington, DC. [Google Scholar]
- 6.Wassermann A, Neisser A, Bruck C. 1906. Eine serodiagnostische Reaktion bei Syphilis. Dtsch Med Wochenschr (Berlin) 32:745–746. [Google Scholar]
- 7.Catterall RD. 1972. Systemic disease and the biological false positive reaction. Br J Vener Dis 48:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portinoy J, Garson W, Smith CA. 1957. Rapid plasma reagin test for syphilis. Public Health Rep 72:761–766. [PMC free article] [PubMed] [Google Scholar]
- 9.Falcone VH, Stout GW, Moore MB Jr. 1964. Evaluation of rapid plasma reagin (circle) card test. Public Health Rep 79:491–495. [PMC free article] [PubMed] [Google Scholar]
- 10.Pettit DE, Larsen SA, Harbec PS, Feeley JC, Parham CE, Cruce DD, Hambie EA, Perryman MW. 1983. Toluidine red unheated serum test, a nontreponemal test for syphilis. J Clin Microbiol 18:1141–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanff PA, Bishop NH, Miller JN, Lovett MA. 1983. Humoral immune response in experimental syphilis to polypeptides of Treponema pallidum. J Immunol 131:1973–1977. [PubMed] [Google Scholar]
- 12.Lukehart SA, Baker-Zander SA, Sell S. 1986. Characterization of the humoral immune response of the rabbit to antigens of Treponema pallidum after experimental infection and therapy. Sex Transm Dis 13:9–15. [DOI] [PubMed] [Google Scholar]
- 13.Müller F, Oelerich S. 1981. Treponema-specific and antilipoidal 19S (IgM) antibodies in penicillin-treated and untreated rabbits after infection with Treponema pallidum. Br J Vener Dis 57:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wende RD, Mudd RL, Knox JM, Holder WR. 1971. The VDRL slide test in 322 cases of darkfield positive primary syphilis. South Med J 64:633–634. [DOI] [PubMed] [Google Scholar]
- 15.Geisler WM. 2004. The prozone phenomenon in syphilis testing. South Med J 97:327–328. doi: 10.1097/01.SMJ.0000092571.52330.13. [DOI] [PubMed] [Google Scholar]
- 16.McMillan A, Young H. 2008. Reactivity in the Venereal Diseases Research Laboratory test and the Mercia IgM enzyme immunoassay after treatment of early syphilis. Int J STD AIDS 19:689–693. doi: 10.1258/ijsa.2008.008104. [DOI] [PubMed] [Google Scholar]
- 17.Seña AC, Wolff M, Martin DH, Behets F, Van Damme K, Leone P, Langley C, McNeil L, Hook EW. 2011. Predictors of serological cure and Serofast State after treatment in HIV-negative persons with early syphilis. Clin Infect Dis 53:1092–1099. doi: 10.1093/cid/cir671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knaute DF, Graf N, Lautenschlager S, Weber R, Bosshard PP. 2012. Serological response to treatment of syphilis according to disease stage and HIV status. Clin Infect Dis 55:1615–1622. doi: 10.1093/cid/cis757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romanowski B, Sutherland R, Fick GH, Mooney D, Love EJ. 1991. Serologic response to treatment of infectious syphilis. Ann Intern Med 114:1005–1009. doi: 10.7326/0003-4819-114-12-1005. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC). 2010. Sexually transmitted diseases guidelines, 2010. MMWR Morb Mortal Wkly Rep 59:1–116. http://www.cdc.gov/std/treatment/2010/std-treatment-2010-rr5912.pdf.20075837 [Google Scholar]
- 21.Nandwani R, Evans DT. 1995. Are you sure it's syphilis? A review of false positive serology. Int J STD AIDS 6:241–248. [DOI] [PubMed] [Google Scholar]
- 22.Ratnam S. 2005. The laboratory diagnosis of syphilis. Can J Infect Dis Med Microbiol 16:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Augenbraun M, French A, Glesby M, Sanchez-Keeland L, Young M, Greenblatt R, Sharma A. 2010. Hepatitis C virus infection and biological false-positive syphilis tests. Sex Transm Infect 86:97–98. doi: 10.1136/sti.2009.040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garson W. 1957. The treponemal tests for syphilis. South Med J 50:911–918. doi: 10.1097/00007611-195707000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Hunter EF, Deacon WE, Meyer PE. 1964. An improved FTA test for syphilis, the absorption procedure (FTA-ABS). Public Health Rep 79:410–412. doi: 10.2307/4592145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter EF. 1975. The fluorescent treponemal antibody-absorption (FTA-ABS) test for syphilis. CRC Crit Rev Clin Lab Sci 5:315–330. [DOI] [PubMed] [Google Scholar]
- 27.Pope V, Fears MB, Morrill WE, Castro A, Kikkert SE. 2000. Comparison of the Serodia Treponema pallidum particle agglutination, Captia Syphilis-G, and SpiroTek Reagin II tests with standard test techniques for diagnosis of syphilis. J Clin Microbiol 38:2543–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebel A, Vanneste L, Cardinaels M, Sablon E, Samson I, De Bosschere K, Hulstaert F, Zrein M. 2000. Validation of the INNO-LIA syphilis kit as a confirmatory assay for Treponema pallidum antibodies. J Clin Microbiol 38:215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagedorn HJ, Kraminer-Hagedorn A, De Bosschere K, Hulstaert F, Pottel H, Zrein M. 2002. Evaluation of INNO-LIA syphilis assay as a confirmatory test for syphilis. J Clin Microbiol 40:973–978. doi: 10.1128/JCM.40.3.973-978.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam TK, Lau HY, Lee YP, Fung SM, Leung WL, Kam KM. 2010. Comparative evaluation of the INNO-LIA syphilis score and the MarDx Treponema pallidum immunoglobulin G Marblot test assays for the serological diagnosis of syphilis. Int J STD AIDS 21:110–113. doi: 10.1258/ijsa.2009.009026. [DOI] [PubMed] [Google Scholar]
- 31.Sambri V, Marangoni A, Eyer C, Reichhuber C, Soutschek E, Negosanti M, D'Antuono A, Cevenini R. 2001. Western immunoblotting with five Treponema pallidum recombinant antigens for serologic diagnosis of syphilis. Clin Diagn Lab Immunol 8:534–549. doi: 10.1128/CDLI.8.3.534-539.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Binnicker MJ, Jespersen DJ, Rollins LO. 2011. Treponema-specific tests for the serodiagnosis of syphilis: a comparative evaluation of seven assays. J Clin Microbiol 49:1313–1317. doi: 10.1128/JCM.02555-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez E, Jespersen DJ, Harring JA, Binnicker MJ. 2010. Evaluation of the Bio-Rad BioPlex 2200 syphilis multiplex flow immunoassay for the detection of IgM- and IgG-class antitreponemal antibodies. Clin Vaccine Immunol 17:966–968. doi: 10.1128/CVI.00086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong EH, Klausner JD, Caguin-Grygiel G, Madayag C, Barber KO, Qiu JS, Liska S, Pandori MW. 2011. Evaluation of an IgM/IgG sensitive enzyme immunoassay and the utility of index values for the screening of syphilis infection in a high-risk population. Sex Transm Dis 38:528–532. doi: 10.1097/OLQ.0b013e318205491a. [DOI] [PubMed] [Google Scholar]
- 35.Donkers A, Levy HR, Letens-van Vliet A. 2014. Syphilis detection using the Siemens ADVIA Centaur Syphilis treponemal assay. Clin Chim Acta 433:84–87. doi: 10.1016/j.cca.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention (CDC). 2011. Discordant results from reverse sequence syphilis screening–five laboratories, United States, 2006-2010. MMWR Morb Mortal Wkly Rep 60:133–137. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6005a1.htm. [PubMed] [Google Scholar]
- 37.Miller JN. 1975. Value and limitations of non-treponemal and treponemal tests in the laboratory diagnosis of syphilis. Clin Obstet Gynecol 18:191–203. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. 1982. Treponemal infections. World Health Organ Tech Rep Ser 674:1–75. [PubMed] [Google Scholar]
- 39.Binnicker MJ. 2012. Which algorithm should be used to screen for syphilis? Curr Opin Infect Dis 25:79–85. doi: 10.1097/QCO.0b013e32834e9a3c. [DOI] [PubMed] [Google Scholar]
- 40.Cole MJ, Perry KR, Parry JV. 2007. Comparative evaluation of 15 serological assays for the detection of syphilis infection. Eur J Clin Microbiol Infect Dis 26:705–713. [DOI] [PubMed] [Google Scholar]
- 41.Egglestone SI, Turner AJ. 2000. Serological diagnosis of syphilis. PHLS Syphilis Serology Working Group. Commun Dis Public Health 3:158–162. [PubMed] [Google Scholar]
- 42.French P, Gomberg M, Janier M, Schmidt B, van Voorst Vader P, Young H. 2009. IUSTI: 2008 European Guidelines on the Management of Syphilis. Int J STD AIDS 20:300–309. doi: 10.1258/ijsa.2008.008510. [DOI] [PubMed] [Google Scholar]
- 43.Loeffelholz MJ, Binnicker MJ. 2012. It is time to use treponema-specific antibody screening tests for diagnosis of syphilis. J Clin Microbiol 50:2–6. doi: 10.1128/JCM.06347-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith BC, Simpson Y, Morshed MG, Cowen LL, Hof R, Wetherell C, Cameron CE. 2013. New proteins for a new perspective on syphilis diagnosis. J Clin Microbiol 51:105–111. doi: 10.1128/JCM.01390-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park IU, Chow JM, Bolan G, Stanley M, Shieh J, Schapiro JM. 2011. Screening for syphilis with the treponemal immunoassay: analysis of discordant serology results and implications for clinical management. J Infect Dis 204:1297–1304. doi: 10.1093/infdis/jir524. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention (CDC). 2008. Syphilis testing algorithms using treponemal tests for initial screening—four laboratories, New York City, 2005-2006. MMWR Morb Mortal Wkly Rep 57:872–875. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5732a2.htm. [PubMed] [Google Scholar]
- 47.Mishra S, Boily MC, Ng V, Gold WL, Okura T, Shaw M, Mazzulli T, Fisman DN. 2011. The laboratory impact of changing syphilis screening from the rapid-plasma regain to a treponemal enzyme immuno assay: a case study from the greater Toronto area. Sex Transm Dis 38:190–196. doi: 10.1097/OLQ.0b013e3181f07e91. [DOI] [PubMed] [Google Scholar]
- 48.Tong ML, Lin LR, Liu LL, Zhang HL, Huang SJ, Chen YY, Guo XJ, Xi Y, Liu L, Chen FY, Zhang YF, Zhang Q, Yang TC. 2014. Analysis of 3 algorithms for syphilis serodiagnosis and implications for clinical management. Clin Infect Dis 58:1116–1124. doi: 10.1093/cid/ciu087. [DOI] [PubMed] [Google Scholar]
- 49.Chuck A, Ohinmaa A, Tilley P, Singh AE, Jacobs P. 2008. Cost effectiveness of enzyme immunoassay and immunoblot testing for the diagnosis of syphilis. Int J STD AIDS 19:393–399. doi: 10.1258/ijsa.2007.007272. [DOI] [PubMed] [Google Scholar]
- 50.Owusu-Edusei K Jr, Peterman TA, Ballard RC. 2011. Serologic Testing of syphilis in the United Sates: a cost-effective analysis of two screening algorithms. Sex Transm Dis 38:1–7. doi: 10.1097/OLQ.0b013e3181ec51f1. [DOI] [PubMed] [Google Scholar]
- 51.Owusu-Edusei K Jr, Koski KA, Ballard RC. 2011. The tale of two serologic tests to screen for syphilis - treponemal and nontreponemal: does the order matter? Sex Transm Dis 38:448–456. doi: 10.1097/OLQ.0b013e3182036a0f. [DOI] [PubMed] [Google Scholar]
- 52.Gratrix J, Plitt S, Lee BE, Ferron L, Anderson A, Verity R, Prasad E, Bunyan R, Zahariadis G, Singh AE. 2012. Impact of reverse sequence syphilis screening on new diagnoses of late latent syphilis in Edmonton, Canada. Sex Transm Dis 39:528–530. doi: 10.1097/OLQ.0b013e31824e53f7. [DOI] [PubMed] [Google Scholar]
- 53.Ward P. 2006. Near-patient testing will improve the control of sexually transmitted infections: control of sexually transmitted infections: the arguments in favour. Sex Transm Infect 82:506–508. doi: 10.1136/sti.2005.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tucker JD, Brown LB, Yin Y-P, Chen X-S, Cohen MS. 2010. Accelerating worldwide syphilis screening through rapid testing: a systematic review. Lancet Infect Dis 10:381–386. doi: 10.1016/S1473-3099(10)70092-X. [DOI] [PubMed] [Google Scholar]
- 55.Public Health Agency of Canada. 2009. Report on sexually transmitted infections in Canada: 2008. http://www.phac-aspc.gc.ca/sti-its-surv-epi/sum-som-eng.php.
- 56.Public Health England. 2013. Health protection report. Sexually transmitted infections and chlamydia in England: 2013. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/345181/Volume_8_number_24_hpr2414_AA_stis.pdf.
- 57.Patton ME, Su JR, Nelson R, Weinstock H; Centers for Disease Control and Prevention (CDC) . 2014. Primary and secondary syphilis–United States, 2005-2013. MMWR Morb Mortal Wkly Rep 63:402–406. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6318a4.htm. [PMC free article] [PubMed] [Google Scholar]
- 58.Greer L, Wendel GD Jr. 2008. Rapid diagnostic methods in sexually transmitted infections. Infect Dis Clin North Am 22:601–617. doi: 10.1016/j.idc.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 59.Kay NS, Peeling RW, Mabey DC. 2014. State of the art syphilis diagnostics: rapid point-of-care tests. Expert Rev Anti Infect Ther 12:63–73. doi: 10.1586/14787210.2014.860356. [DOI] [PubMed] [Google Scholar]
- 60.Jafari Y, Peeling RW, Shivkumar S, Claessens C, Joseph L, Pai NP. 2013. Are Treponema pallidum specific rapid and point-of-care tests for syphilis accurate enough for screening in resource limited settings? Evidence from a meta-analysis. PLoS One 8:e54695. doi: 10.1371/journal.pone.0054695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Causer LM, Kaldor JM, Fairley CK, Donovan B, Karapanagiotidis T, Leslie DE, Robertson PW, McNulty AM, Anderson D, Wand H, Conway DP, Denham I, Ryan C, Guy RJ. 2014. A laboratory-based evaluation of four rapid point-of-care tests for syphilis. PLoS One 11:e91504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaydos C, Hardick J. 2014. Point of care diagnostics for sexually transmitted infections: perspectives and advances. Expert Rev Anti Infect Ther 12:657–672. doi: 10.1586/14787210.2014.880651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montoya PJ, Lukehart SA, Brentlinger PE, Blanco AJ, Floriano F, Sairosse J, Gloyd S. 2006. Comparison of the diagnostic accuracy of a rapid immunochromatographic tests and the rapid plasma reagin test for antenatal syphilis screening in Mozambique. Bull World Health Organ 84:97–104. doi: 10.2471/BLT.04.018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benzaken AS, Sabido M, Galban EG, Pedroza V, Vasquez F, Araújo A, Peeling RW, Mayaud P. 2008. Field evaluation of the performance and testing costs of a rapid point-of-care test for syphilis in a red-light district of Manaus, Brazil. Sex Transm Infect 84:297–302. doi: 10.1136/sti.2007.029462. [DOI] [PubMed] [Google Scholar]
- 65.Terris-Prestholt F, Watson-Jones D, Mugeye K, Kumaranayake L, Ndeki L, Weiss H, Changalucha J, Todd J, Lisekie F, Gumodoka B, Mabey D, Hayes R. 2003. Is antenatal syphilis screening still cost effective in sub-Saharan Africa. Sex Transm Infect 79:375–381. doi: 10.1136/sti.79.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levin CE, Steele M, Atherly D, García SG, Tinajeros F, Revollo R, Richmond K, Díaz-Olavarrieta C, Martin T, Floriano F, Massango I, Gloyd S. 2007. Analysis of operational costs of using rapid syphilis tests for the detection of maternal syphilis in Bolivia and Mozambique. Sex Transm Dis 34:S47–54. doi: 10.1097/01.olq.0000245986.62775.b6. [DOI] [PubMed] [Google Scholar]
- 67.Rydzak CE, Goldie SJ. 2008. Cost-effectiveness of rapid point-of-care prenatal syphilis screening in sub-Saharan Africa. Sex Transm Dis 35:775–784. doi: 10.1097/OLQ.0b013e318176196d. [DOI] [PubMed] [Google Scholar]
- 68.Owusu-Edusei K Jr, Gift T, Ballard R. 2011. Cost-effectiveness of a dual non-treponemal/treponemal syphilis point-of-care test to prevent adverse pregnancy outcomes in sub-Saharan Africa. Sex Transm Dis 38:997–1003. doi: 10.1097/OLQ.0b013e3182260987. [DOI] [PubMed] [Google Scholar]
- 69.Castro AR, Esfandiari J, Kumar S, Ashton M, Kikkert SE, Park MM, Ballard RC. 2010. Novel point-of-care test for the simultaneous detection of nontreponemal and treponemal antibodies in patients with syphilis. J Clin Microbiol 48:4615–4619. doi: 10.1128/JCM.00624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yin YP, Chen XS, Wei WH, Gong KL, Cao WL, Yong G, Feng L, Huang SJ, Wang DM, Han Y, Chen SC, Mabey D, Peeling RW. 2013. A dual point-of-care test shows good performance in simultaneously detecting nontreponemal and treponemal antibodies in patients with syphilis: a multisite evaluation study in China. Clin Infect Dis 56:659–665. doi: 10.1093/cid/cis928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Campos PE, Buffardi Al, Chiappe M, Buendia C, Garcia PJ, Carcamo CP, Garnett G, White P, Holmes KK. 2006. Utility of the Determine Syphilis TP rapid test in commercial sex venues in Peru. Sex Transm Infect 82(Suppl 5):v22–v25. doi: 10.1136/sti.2006.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herring A, Ballard R, Pope V, Adegbola RA, Changalucha J, Fitzgerald DW, Hook EW III, Kubanova A, Mananwatte S, Pape JW, Sturm AW, West B, Yin YP, Peeling RW. 2006. A multi-centre evaluation of nine rapid, point-of-care syphilis tests using archived sera. Sex Transm Infect 82:v7–v12. doi: 10.1136/sti.2006.022707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lukehart SA, Hook EW III, Baker-Zander SA, Collier AC, Critchlow CW, Handsfield HH. 1988. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann Intern Med 109:855–862. doi: 10.7326/0003-4819-109-11-855. [DOI] [PubMed] [Google Scholar]
- 74.Marra CM. 2009. Update on neurosyphilis. Curr Infect Dis Rep 11:127–134. doi: 10.1007/s11908-009-0019-1. [DOI] [PubMed] [Google Scholar]
- 75.Golden MR, Marra CM, Holmes KK. 2003. Update on syphilis: resurgence of an old problem. JAMA 290:1510–1514. doi: 10.1001/jama.290.11.1510. [DOI] [PubMed] [Google Scholar]
- 76.O'donnell JA, Emery CL. 2005. Neurosyphilis: a current review. Curr Infect Dis Rep 7:277–284. doi: 10.1007/s11908-005-0060-7. [DOI] [PubMed] [Google Scholar]
- 77.Ghanem KG. 2010. Review: neurosyphilis: a historical perspective and review. CNS Neurosci Ther 16:e157–168. doi: 10.1111/j.1755-5949.2010.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Becker GD. 1979. Late syphilis hearing loss: a diagnostic and therapeutic dilemma. Laryngoscope 89:1273–1288. [DOI] [PubMed] [Google Scholar]
- 79.Marra CM, Maxwell CL, Smith SL, Lukehart SA, Rompalo AM, Eaton M, Stoner BP, Augenbraun M, Barker DE, Corbett JJ, Zajackowski M, Raines C, Nerad J, Kee R, Barnett SH. 2004. Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features. J Infect Dis 189:369–376. doi: 10.1086/381227. [DOI] [PubMed] [Google Scholar]
- 80.Libois A, De Wit S, Poll B, Garcia F, Florence E, Del Rio A, Sanchez P, Negredo E, Vandenbruaene M, Gatell JM, Clumeck N. 2007. HIV and syphilis: when to perform a lumbar puncture. Sex Transm Dis 34:141–144. doi: 10.1097/01.olq.0000230481.28936.e5. [DOI] [PubMed] [Google Scholar]
- 81.Ghanem KG, Moore RD, Rompalo AM, Erbelding EJ, Zenilman JM, Gebo KA. 2009. Lumbar puncture in HIV-infected patients with syphilis and no neurologic symptoms. Clin Infect Dis 48:816–821. doi: 10.1086/597096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hay PE, Clarke JR, Strugnell RA, Taylor-Robinson D, Goldmeier D. 1990. Use of the polymerase chain reaction to detect DNA sequences specific to pathogenic treponemes in cerebrospinal fluid. FEMS Microbiol Lett 56:233–238. [DOI] [PubMed] [Google Scholar]
- 83.Burstain JM, Grimpel E, Lukehart SA, Norgard MV, Radolf JD. 1991. Sensitive detection of Treponema pallidum by using the polymerase chain reaction. J Clin Microbiol 29:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Larsen S, Johnson RE. 1998. Diagnostic tests. http://www.cdc.gov/std/syphilis/manual-1998/chapt1.pdf.
- 85.Marra CM, Tantalo LC, Maxwell CL, Ho EL, Sahi SK, Jones T. 2012. The rapid plasma reagin test cannot replace the venereal disease research laboratory test for neurosyphilis diagnosis. Sex Transm Dis 39:453–457. doi: 10.1097/OLQ.0b013e31824b1cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jaffe HW, Larsen SA, Peters M, Jove DF, Lopez B, Schroeter AL. 1978. Tests for treponemal antibody in CSF. Arch Intern Med 138:252–255. [PubMed] [Google Scholar]
- 87.Harding AS, Ghanem KG. 2012. The performance of cerebrospinal fluid treponemal-specific antibody tests in neurosyphilis: a systematic review. Sex Transm Dis 39:291–297. doi: 10.1097/OLQ.0b013e31824c0e62. [DOI] [PubMed] [Google Scholar]
- 88.Berger JR, Dean D. 2014. Neurosyphilis. Handb Clin Neurol 121:1461–1472. doi: 10.1016/B978-0-7020-4088-7.00098-5. [DOI] [PubMed] [Google Scholar]
- 89.van Eijk RVW, Wolters EC, Tutuarima JA, Hische EAH, Bos JD, van Trotsenburg L, de Koning GAJ, van der Helm HJ. 1987. Effect of early and late syphilis on central nervous system: cerebrospinal fluid changes and neurological deficit. Genitourin Med 63:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Castro R, Prieto ES, Aguas MJ, Manata MJ, Botas J, Araujo C, Borges F, Aldir I, Exposto FL. 2006. Evaluation of the Treponema pallidum particle agglutination technique (TPPA) in the diagnosis of neurosyphilis. J Clin Lab Anal 20:233–238. doi: 10.1002/jcla.20147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marra CM, Critchlow CW, Hook EW III, Collier AC, Lukehart SA. 1995. Cerebrospinal fluid treponemal antibodies in untreated early syphilis. Arch Neurol 52:68–72. [DOI] [PubMed] [Google Scholar]
- 92.Luger A, Schmidt BL, Steyrer K, Schonwald ES. 1981. Diagnosis of neurosyphilis by examination of the cerebrospinal fluid. Br J Vener Dis 57:232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stoner B. 2007. Current controversies in the management of adult syphilis. Clin Infect Dis 44:S130–S146. doi: 10.1086/511426. [DOI] [PubMed] [Google Scholar]
- 94.Johnson PD, Graves SR, Stewart L, Warren R, Dwyer B, Lucas RC. 1991. Specific syphilis serological tests may become negative in HIV infection. AIDS 5:419–423. doi: 10.1097/00002030-199104000-00010. [DOI] [PubMed] [Google Scholar]
- 95.Katz DA, Berger JR, Duncan RC. 1993. Neurosyphilis. A comparative study of the effects of infection with human immunodeficiency virus. Arch Neurol 50:243–249. [DOI] [PubMed] [Google Scholar]
- 96.Dattner B, Thomas EW, De Mello L. 1951. Criteria for the management of neurosyphilis. Am J Med 10:463–467. [DOI] [PubMed] [Google Scholar]
- 97.Jordan KG. 1988. Modern neurosyphilis — a critical analysis. West J Med 149:47–57. [PMC free article] [PubMed] [Google Scholar]
- 98.Flores JL. 1995. Syphilis. A tale of twisted treponemes. West J Med 163:552–559. [PMC free article] [PubMed] [Google Scholar]
- 99.Marra CM, Longstreith WT Jr, Maxwell CL, Lukehart SA. 1996. Resolution of serum and cerebrospinal fluid abnormalities after treatment of neurosyphilis. Influence of concomitant human immunodeficiency virus infection. Sex Transm Dis 23:184–189. [DOI] [PubMed] [Google Scholar]
- 100.Marra CM, Maxwell CL, Tantalo LC, Sahi SK, Lukehart SA. 2008. Normalization of serum rapid plasma reagin titer predicts normalization of cerebrospinal fluid and clinical abnormalities after treatment of neurosyphilis. Clin Infect Dis 47:893–899. doi: 10.1086/591534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hawkes S, Matin N, Broutet N, Low N. 2011. Effectiveness of interventions to improve screening for syphilis in pregnancy: a systematic review and meta-analysis. Lancet Infect Dis 11:684–691. doi: 10.1016/S1473-3099(11)70104-9. [DOI] [PubMed] [Google Scholar]
- 102.Walker DG, Walker GJ. 2002. Forgotten but not gone: the continuing scourge of congenital syphilis. Lancet Infect Dis 2:432–436. doi: 10.1016/S1473-3099(02)00319-5. [DOI] [PubMed] [Google Scholar]
- 103.Singh AE, Sutherland K, Lee B, Robinson JL, Wong T. 2007. Resurgence of early congenital syphilis in Alberta. CMAJ 177:33–36. doi: 10.1503/cmaj.070495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dorfman DH, Glaser JH. 1990. Congenital syphilis presenting in infants after the newborn period. N Engl J Med 323:1299–1302. doi: 10.1056/NEJM199011083231902. [DOI] [PubMed] [Google Scholar]
- 105.Jonna S, Collins M, Abedin M, Young M, Milteer R, Beeram M. 1995. Postneonatal screening for congenital syphilis. J Fam Pract 41:286–288. [PubMed] [Google Scholar]
- 106.Woods CR. 2009. Congenital syphilis-persisting pestilence. Pediatr Infect Dis J 28:536–537. doi: 10.1097/INF.0b013e3181ac8a69. [DOI] [PubMed] [Google Scholar]
- 107.Stoll BJ. 1994. Congenital syphilis: evaluation and management of neonates born to mothers with reactive serologic tests for syphilis. Pediatr Infect Dis J 13:845–852. [PubMed] [Google Scholar]
- 108.Genç M, Ledger WJ. 2000. Syphilis in pregnancy. Sex Transm Infect 76:73–79. doi: 10.1136/sti.76.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schmitz JL, Gertis KS, Mauney C, Stamm LV, Folds JD. 1994. Laboratory diagnosis of congenital syphilis by immunoglobulin M (IgM) and IgA immunoblotting. Clin Diagn Lab Immunol 1:32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rawstron SA, Mehta S, Marcellino L, Rempel J, Chery F, Bromberg K. 2001. Congenital syphilis and fluorescent treponemal antibody test reactivity after the age of 1 year. Sex Transm Dis 28:412–416. doi: 10.1097/00007435-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 111.Stoll BJ, Lee FK, Larsen S, Hale E, Schwartz D, Rice RJ, Ashby R, Holmes R, Nahmias AJ. 1993. Clinical and serologic evaluation of neonates for congenital syphilis: a continuing diagnostic dilemma. J Infect Dis 167:1093–1099. doi: 10.1093/infdis/167.5.1093. [DOI] [PubMed] [Google Scholar]
- 112.Hollier LM, Harstad TW, Sanchez PJ, Twickler DM, Wendel GD. 2001. Fetal syphilis: clinical and laboratory characteristics. Obstet Gynecol 97:947–953. doi: 10.1016/S0029-7844(01)01367-9. [DOI] [PubMed] [Google Scholar]
- 113.Meyer MP, Roditi D, Louw S. 1992. IgM rheumatoid factor removal and performance of the FTA-ABS (IgM) test in congenital syphilis. Genitourin Med 68:249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Herremans M, Notermans DW, Mommers M, Kortbeek LM. 2007. Comparison of a Treponema pallidum IgM immunoblot with a 19S fluorescent treponemal antibody absorption test for the diagnosis of congenital syphilis. Diagn Microbiol Infect Dis 59:61–66. doi: 10.1016/j.diagmicrobio.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 115.Sánchez PJ, Wendel GD, Norgard MV. 1991. Congenital syphilis associated with negative results of maternal serologic tests at delivery. Am J Dis Child 145:967–969. [DOI] [PubMed] [Google Scholar]
- 116.Herremans T, Kortbeek L, Notermans DW. 2010. A review of diagnostic tests for congenital syphilis in newborns. Eur J Clin Microbiol Infect Dis 29:495–501. [DOI] [PubMed] [Google Scholar]
- 117.Singh AE, Guenette T, Gratrix J, Bergman J, Parker P, Anderson B, Plitt S, Lee B, Robinson J. 2013. Seroreversion of treponemal tests in infants meeting Canadian surveillance criteria for confirmed early congenital syphilis. Pediatr Infect Dis J 32:199–202. doi: 10.1097/INF.0b013e318273599c. [DOI] [PubMed] [Google Scholar]
- 118.Schulte JM, Burkham S, Hamaker D, St Louis ME, Paffel JM, Hutcheson D, Caldwell MB, Dominguez KL, Levine WC. 2001. Syphilis among HIV-infected mothers and their infants in Texas from 1988 to 1994. Sex Transm Dis 28:315–320. doi: 10.1097/00007435-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 119.Lee MJ, Hallmark RJ, Frenkel LM, Del Priore G. 1998. Maternal syphilis and vertical perinatal transmission of human immunodeficiency virus type-1 infection. Int J Gynaecol Obstet 63:247–252. doi: 10.1016/S0020-7292(98)00165-9. [DOI] [PubMed] [Google Scholar]