Figure 2.
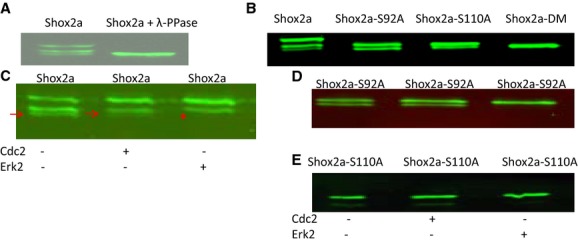
Shox2a is phosphorylated on serine residues in vivo. A, Western blotting assay shows the presence of shifted forms of Shox2a that are eliminated by incubation of Shox2a proteins with lambda protein phosphatase (λ‐PPase). B, Serine to alanine mutation on Ser92 (Shox2a‐S92A), Ser110 (Shox2a‐S110A), or both sites (Shox2a‐DM) eliminates phosphorylated Shox2a forms. Treatment of immunoprecipitated Shox2a (C), Shox2a‐S92A (D), or Shox2a‐110A (E) by Cdc2 or Erk2 demonstrates that Shox2a can be phosphorylated by Erk2, but not Cdc2. Red arrows point to the unphosphorylated band and red start indicates the disappearance of unphopshorylated band after Erk2 treatment in (C). Cdc2 indicates cell division control protein 2; Erk1/2, extracellular signal‐regulated kinase 1 and 2.
