Figure 7.
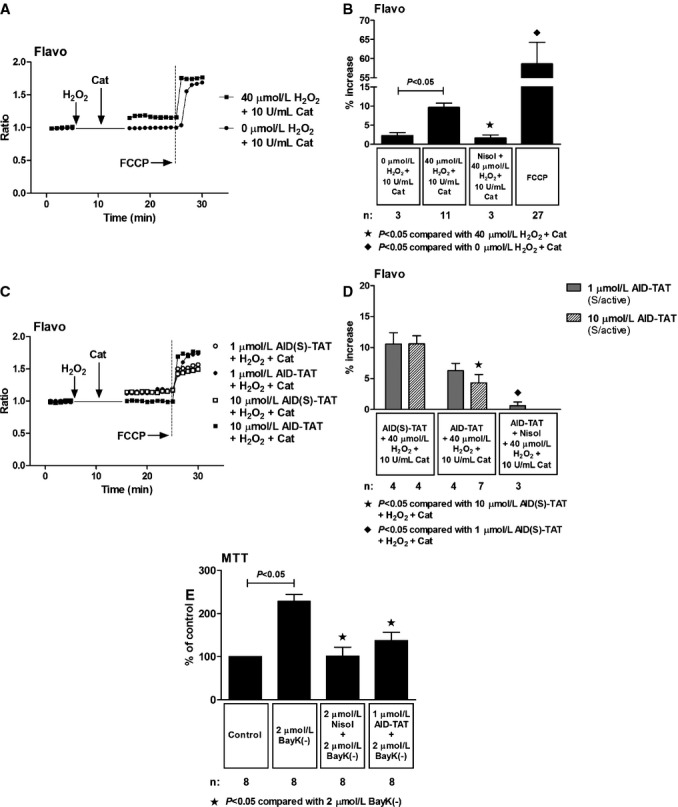
Effect of AID‐TAT peptide on flavoprotein oxidation in vitro. A, Representative traces of flavoprotein autofluorescence recorded in a myocyte before and after 5 minutes of exposure to 40 μmol/L of H2O2 followed by 5 minutes of exposure to 10 U/mL of catalase and a myocyte before and after 5 minutes of exposure to 0 μmol/L of H2O2 followed by 5 minutes of exposure to 10 U/mL of catalase. Vertical arrows indicate when drugs were added. Carbonyl cyanide‐4‐(trifluoromethoxy)phenylhydrazone (FCCP; 10 μmol/L) was added to increase flavoprotein signal, confirming the signal was mitochondrial in origin. B, Mean±SEM of changes flavoprotein autofluorescence for all myocytes exposed to 40 μmol/L of H2O2, 10 U/mL of catalase, 10 μmol/L of nisoldipine (Nisol), and 10 μmol/L of FCCP as indicated. C, Representative traces of flavoprotein autofluorescence recorded in myocytes before and after 5 minutes of exposure to 40 μmol/L of H2O2 followed by 5 minutes of exposure to 10 U/mL of catalase in the presence of either 1 or 10 μmol/L of AID(S)‐TAT or AID‐TAT peptide derived against the AID of the L‐type calcium channel as indicated. Vertical arrows indicate when drugs were added. FCCP (10 μmol/L) was added to increase flavoprotein signal, confirming the signal was mitochondrial in origin. D, Mean±SEM of changes in flavoprotein autofluorescence for all myocytes exposed to 40 μmol/L of H2O2, 10 U/mL of catalase, 1 or 10 μmol/L of AID(S)‐TAT or AID‐TAT peptide, and 10 μmol/L of nisoldipine (Nisol) as indicated. Statistical significance was determined using the Kruskal‐Wallis test followed by Dunn's multiple comparison test. E, Exposure of guinea pig myocytes to BayK(–) causes a significant increase in formation of formazan from tetrozolium salt that can be attenuated by nisoldipine and by AID‐TAT peptide.19 AID indicates alpha‐interacting domain; TAT, transactivator of transcription.
