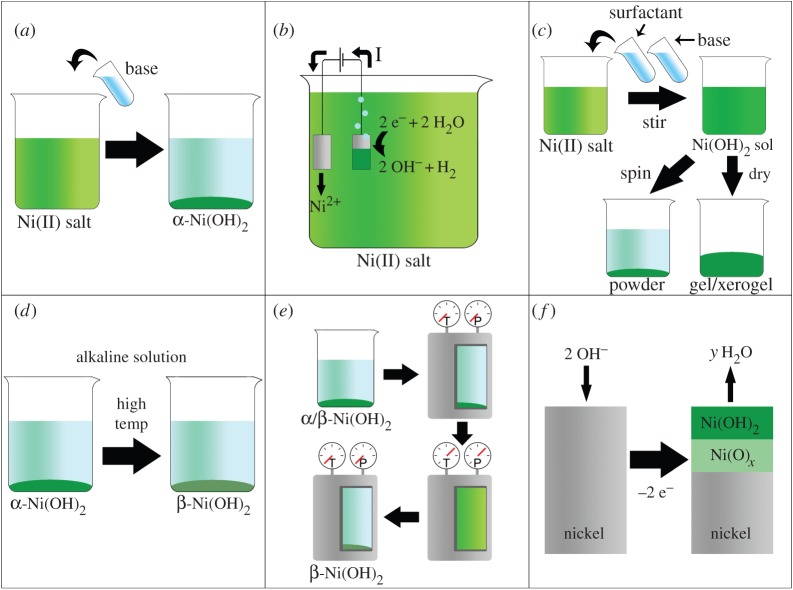
Figure 8.
Six methods to prepare Ni(OH)2. (a) The basification of an aqueous nickel(II) salt solution (e.g. addition of KOH(aq) to NiCl2(aq)). (b) Electrochemical precipitation onto a conductive substrate. The reduction of water produces hydroxide anions at the cathode surface, which react with the nickel(II) cations in solution. In this example, the anode material is a Ni sheet. (c) Sol–gel methods. A solution of a nickel(II) salt and a surfactant (e.g. sodium dodecylsulfate) is basified to form a Ni(OH)2 sol. The sol is treated (e.g. by centrifugation or evaporation) to produce a gel. (d) Chemical ageing converts α-Ni(OH)2 to β-Ni(OH)2. This is typically performed in concentrated alkaline media at high temperatures but also proceeds slowly in room temperature water. (e) The hydrothermal method. α/β-Ni(OH)2 precursor is dissolved in a pressure vessel at high temperatures. The temperature is subsequently decreased and β-Ni(OH)2 precipitates. (f) α/β-Ni(OH)2 surface layers form on nickel and nickel-based alloys as corrosion products or electrochemically formed surface layers, often underlaid by non-stoichiometric nickel oxide. Reproduced with permission from [115]. (Online version in colour.)