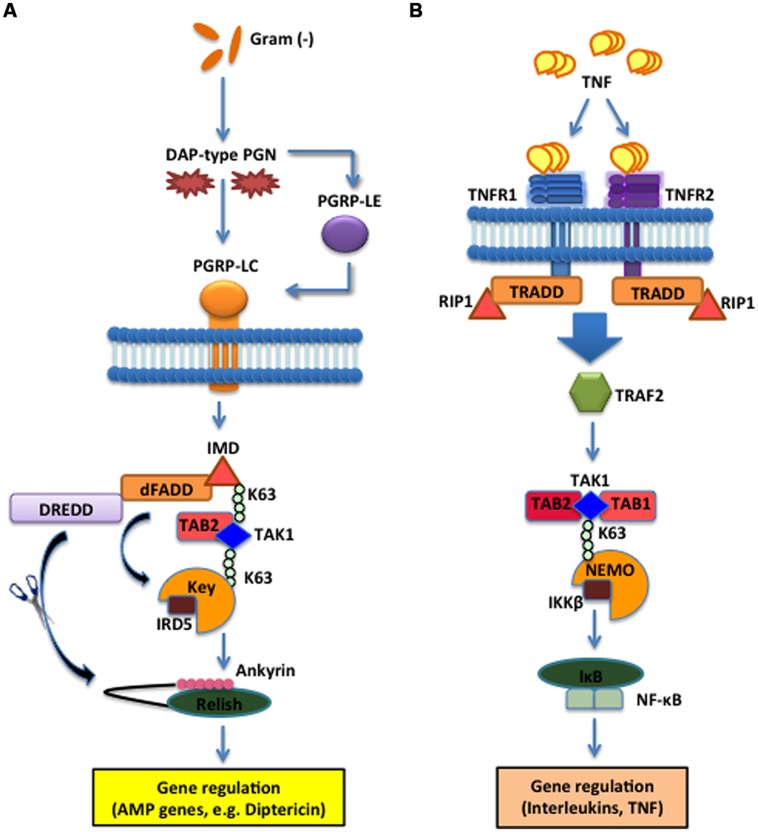
FIGURE 2.
The Imd pathway in the fruit fly and the TNF pathway in the mouse. (A) The D. melanogaster Imd signaling pathway is activated upon direct binding between PGRP-LC and meso-diaminopimelic acid (DAP)-type PG of Gram-negative bacteria and certain Gram-positive bacilli. The intracellular adaptor protein Immune deficiency (Imd) interacts with the Drosophila Fas-associated death domain (dFADD) and the Death related ced-3/Nedd2-like caspase (DREDD) that cleaves Imd, which is then activated by K63-ubiquitination. This leads to the activation of the TAK1 and TAB2 complex that in turn activates the IKK signalosome, which is composed of Immune Response Deficient 5 (IRD5) and Kenny (Key). Relish is subsequently cleaved by DREDD. As a result, the Rel DNA-binding domain is released from the C-terminal ankyrin-repeat/IκB-like domain, and translocates to the nucleus to induce transcription of antimicrobial peptide (AMP) genes, such as Diptericin. (B) In Mus musculus, TNF trimers bind and activate the transmembrane receptors R1 and R2 (TNFR1 and TNFR2) that recruit Tumor necrosis factor receptor type 1-associated DEATH domain protein (TRADD), receptor-interacting protein 1 (RIP1) and TNF receptor-associated factor 2 (TRAF2). The latter employs the Transforming Growth Factor beta (TGF-β) activated kinase 1 (TAK1) (whose activity is directly regulated by K63-linked polyubiquitination) and TAB1 and TAB2 complex to phosphorylate and activate the IKK signalosome, which phosphorylates IκB that dissociates from NF-κB. NF-κB translocates to the nucleus to induce expression of several genes that participate in inflammation and immunity.