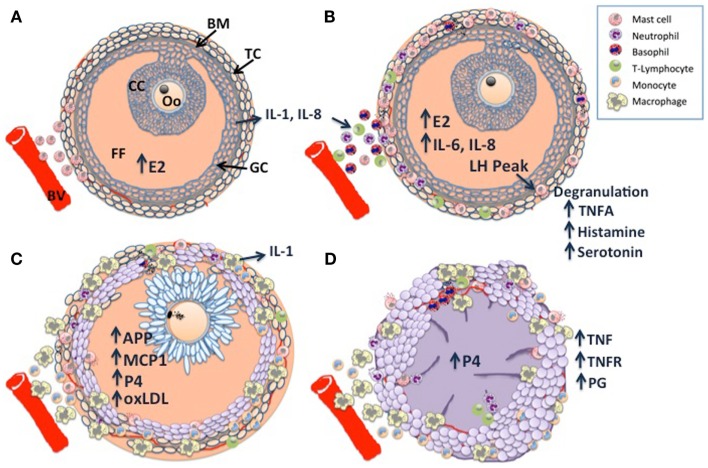
Figure 1.
Schematic diagram of dominant follicle differentiation and corpus luteum formation: follicle differentiation and luteinization appear to be characterized by three waves of immune cell infiltration. (A) Mast cell infiltration of the thecal cell layer (TC) triggered by increasing estradiol (E2) concentrations, note the intact basement membrane (BM) separating the follicle granulosa-cell layer (GC) and follicle contents [cumulus cell layer (CC), surrounding the oocyte (Oo), and the follicular fluid FF] from the ovarian stroma. (B) A peak in luteinizing hormone (LH) pulsatility triggers mast cell degranulation which stimulates the second wave through the direct and indirect actions of TNF-alpha (TNFA), a constituent of the granules. (C) The last wave is characterized by macrophage infiltration, possibly in response to E2 and other chemoattractants such as monocyte chemoattractant protein 1 (MCP1), acute phase proteins (APP), and GC derived oxidized low density lipoprotein (oxLDL). Note the expanded cumulus cells surrounding the metaphase II stage oocyte, there is a switch from E2 synthesis to progesterone synthesis as the follicular cells become luteinized. (D) Following ovulation, granulocytes, neutrophils, and eosinophils constitute the majority of immune cells within the developing corpus luteum (CL), with further infiltration of macrophages and endothelial cells as development and vascularization proceed. Macrophage derived tumor necrosis factor (TNF) is a potent stimulator of luteal prostaglandins (PG), including PGF2a, PGE2, and PG112, which in concert with TNF drive CL vascularization. Large luteal cells derived primarily from the granulosa cells produce the >80% of P4.