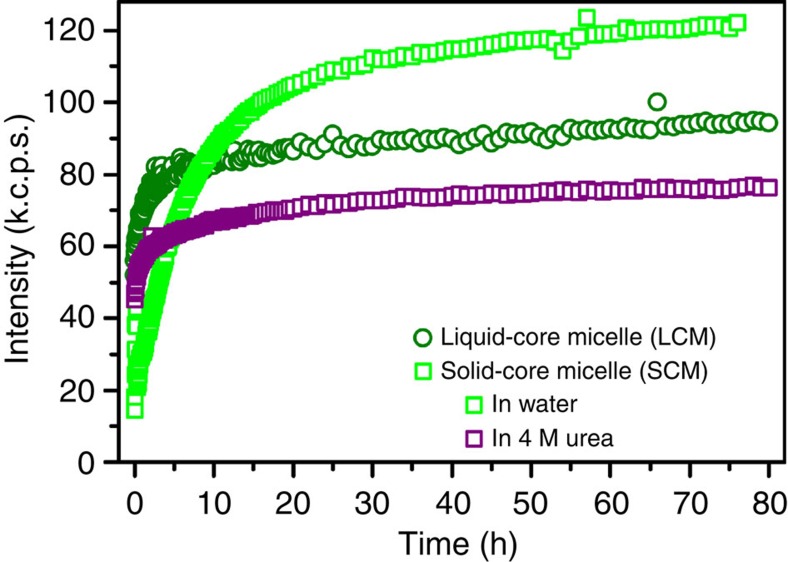
Figure 5. Kinetics of micelle formation.
Total scattered intensity as a function of time for LCMs (circles) and SCMs (squares) depicting the timescale for equilibration. LCMs in water equilibrate relatively quickly, reaching 72% of their equilibrium value within 1 h, while SCMs in water show much slower kinetics, on the order of days; only reaching 25% of their equilibrium value in 1 h. However, the addition of 4 M urea to a SCM sample to disrupt hydrogen bonding between the polypeptides enables fast equilibration, similar to that of LCMs (74% of equilibrium value in 1 h). Micelles were prepared at a polymer concentration of 0.01 mM total polymer concentration using a polyethylene glycol-pLE block copolymer with an average N=50 and either pLK with N=100 for SCMs or p(D,L)K with N=100 for LCMs.