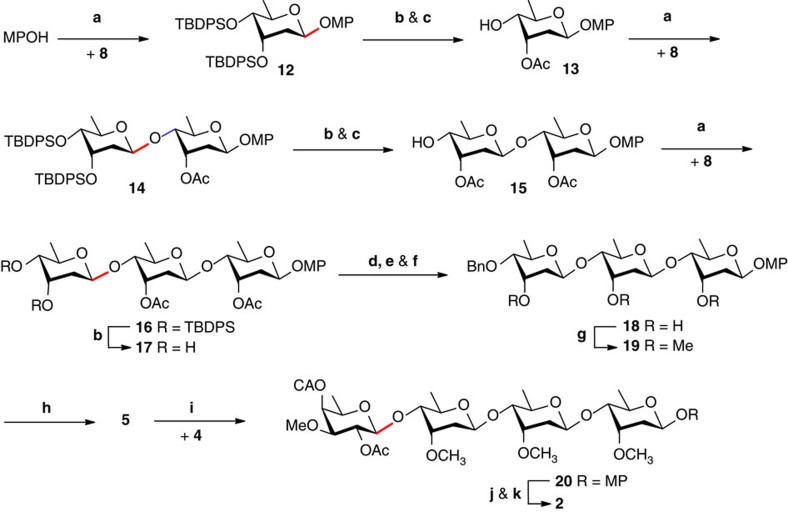
Figure 2. Synthesis of tetrasaccharide donor 2.
Highlighted in red are the nascent glycosidic bonds. (a) Ph3PAuNTf2 (0.1 equiv), toluene, 4 Å MS, −40 °C; 95% and β only (for 12); 99% and β only (for 14 and 16); (b) TBAF, THF, 0 °C to RT; 99% (from 12); 94% (from 14); 96% (from 16); (c) CH3(OMe)3, p-TsOH, RT, 93% for (13); 91% (for 15); (d) nBu2SnO, MeOH, reflux; (e) BnBr, DMF, CsF, RT; (f) LiOH, THF, H2O, RT; 87% (for three steps); (g) MeI, NaH, DMF, 0 °C to RT, 99%; (h) Pd(OH)2/C, H2 (1 atm), Et3N, EtOAc, MeOH, 50 °C, 93%; (i) Ph3PAuNTf2 (0.2 equiv), toluene, 5 Å MS, −50 °C to RT, 93%, β/α=4/1; (j) Ag(DPAH)2, CH3CN, H2O, 0 °C to RT, 95%; (k) o-cyclopropylethynylbenzoic acid, EDCI, DMAP, 4 Å MS, CH2Cl2, RT, 99%. DMAP, 4,4-dimethylaminopyridine; DPAH, bis(hydrogen dipicolinate); EDCI, N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride; MS, molecular sieves; TBAF, tetrabutylammonium fluoride.