Abstract
Background
The routine application of neoadjuvant chemoradiotherapy for T3N0 rectal cancer remains controversial. The aim of this study was to use clinical, Magnetic resonance imaging, and pathological parameters to identify a subgroup of patients with low risk of local recurrence who might be precluded from neoadjuvant chemoradiotherapy.
Methods
We retrospectively reviewed a prospectively maintained database of consecutive rectal cancer patients who underwent curative resection. 166 pathologic confirmed T3N0 rectal cancer patients with tumor located 5–12cm above the anal verge and preoperative circumferential resection margin>1mm were included in analysis. The primary outcomes measured were3- and 5-year local recurrence rates.
Results
Local recurrence was demonstrated during follow-up in 5 patients; the actuarial overall 3- and 5-year local recurrence rates were 2.5% and 3.4%, respectively. Inadequate sampling of lymph nodes (≤12) was associated with higher local recurrence (P = 0.03) in this group of patients.
Conclusion
For upper and middle T3N0 rectal cancer with preoperative circumferential resection margin>1mm, local recurrence rate after total mesorectal excision is low and surgery alone may be enough for this group of patients.
Introduction
The current standard therapy for locally advanced rectal cancer is neoadjuvant chemoradiotherapy (CRT) followed by total mesorectal excision (TME) [1]. However, several studies have reported that local recurrence can be well controlled at a relatively low level (4–8%) by surgery alone in patients with T3N0 rectal cancer, suggesting that neoadjuvant CRT might not be necessary for these patients [2–5]. However, not all patients with T3N0 can be spared from neoadjuvant CRT. Risk of local recurrence is also significantly associated with several other factors like location of tumor [6] and circumferential resection margin (CRM) status [7, 8], which should also be taken into account when determine the necessity of neoadjuvant CRT. The European Society for Medical Oncology (ESMO) guideline for treatment of rectal cancer recommends a flexible strategy on application of neoadjuvant therapy basing on clinical staging, location of tumor, and risk of CRM involvement [9], although the evidence is still limited.
Since neoadjuvant CRT only reduces risk of local recurrence but not distant metastases [10], and inevitably results in short-term and long-term toxicities [11], some studies questioned the strategy of routine application of neoadjuvant CRT to patients with T3N0 rectal cancer, and proposed that only those at high risk of local recurrence should be treated with neoadjuvant CRT [2, 12].
In this study, we used clinical, Magnetic resonance imaging (MRI), and pathological parameters to identify patients with low risk of local recurrence who might be precluded from neoadjuvant CRT.
Materials and Methods
Patient Selection
Patients were identified from a prospective maintained database in the Sun Yat-sen University Cancer Center from January 2005 to December 2010. The study was performed following approval by the ethic committee of Sun Yat-Sen University Cancer Center. We were replied that it’s not necessary to get signatures of patients’ informed consent forms according to the current Chinese medical regulations. The process of the whole study is retrospective, non-invasive, and without any patients’ benefit hurt. Ethics committees approved this consent procedure. The inclusion criteria were as follows: (1) pathologically confirmed T3N0 rectal adenocarcinoma; (2) tumor located 5–12cm above the anal verge; (3) underwent complete curative resection according to the principles of TME; (4) preoperative CRM >1mm in MRI; The exclusion criteria were as follows: (1) patients received neoadjuvant therapy; (2) existence of distant metastases; (3) history of a second primary malignancy. Follow-up, primarily obtained from the institution database, was updated by clinical chart review, physician records, patient correspondence, and telephone interviews.
Treatment Scheme
Preoperative evaluation included history/physical, rigid proctoscopy, colonoscopy, chest x-ray or computed tomography (CT) scan, CT scan of the abdomen, endorectal ultrasound, MRI of the pelvis, and serum carcinoembryonic antigen (CEA) levels. All patients received radical anterior resection according to the principles of TME. All patients were followed at 3-month intervals during the first 2 years after surgery and every 6-month thereafter for an additional period of 3 years. Colonoscopy was done one year after surgery. Ultrasonography of the liver was carried out every 3 months. CT scans of chest, abdomen, and pelvis were performed every year for 5 years. Other investigations were performed when clinically indicated during follow-up.
Evaluation of CRM Status on MRI
CRM involvement was evaluated on pre-operative MRI [13]. The evaluation was done retrospectively by one radiologist who was blinded to the pathology staging. Only patients with mesorectal fascia>1mm were included in this study.
Statistics
Characteristics were described in terms of frequency for the categorical variables and medians for non-normally distributed continuous. Significance was set at P< 0.05. Primary study end points included 3- and 5-year local recurrence rates, relapse free survival (RFS), and disease-specific survival (DSS). Local recurrence was defined as recurrence in the pelvis whether newly diagnosed distant metastases were present or not. Local recurrence and patient survival rates were calculated using the Kaplan-Meier method (with log-rank test). Statistical analyses were performed using the Statistical Package for the Social Sciences program (SPSS Inc. Chicago, IL, version 15.0 for Windows).
Results
Demographic and Clinical Pathologic Characteristics
A total of 166 patients were included. Patient and tumor characteristics were listed in Table 1. The median age at diagnosis was 60 years (range, 27 to 86 years). The median number of dissected lymph nodes was 14 (range, 2 to 34). Median distance from the surgical margin was 3.0 cm (range, 1.0 to 7.0 cm).
Table 1. Demographic and clinicalpathologic characteristics.
| Variable | |
|---|---|
| Total No. of patients | 166 |
| Gender, no. (%) | |
| Male | 111 (66.9) |
| Female | 55 (33.1) |
| Age at diagnosis, median (range), y | 60 (27~86) |
| Distance to anal verge, median(range), cm | 7 (5~12) |
| Grade, no. (%) | (n = 163) |
| Ⅰ | 4 (2.5) |
| Ⅱ | 147 (90.2) |
| Ⅲ | 12 (7.3) |
| Mucin production, no. (%) | |
| Present | 6 (3.6) |
| Absent | 160 (96.4) |
| Preoperative serum CEA level, no. (%) | (n = 164) |
| ≤5 g/ml | 112 (68.3) |
| >5 g/ml | 52 (31.7) |
| Distance from the distal margin, no. (%) | (n = 151) |
| ≤ 2cm | 40 (26.5) |
| >2cm | 111 (73.5) |
| No. of lymph nodes retrieved, no. (%) | |
| ≤12 | 60 (36.1) |
| >12 | 106 (63.9) |
| Adjuvant chemotherapy, no. (%) | |
| No | 71 (42.8) |
| Yes | 95 (57.2) |
Follow-up and Survival
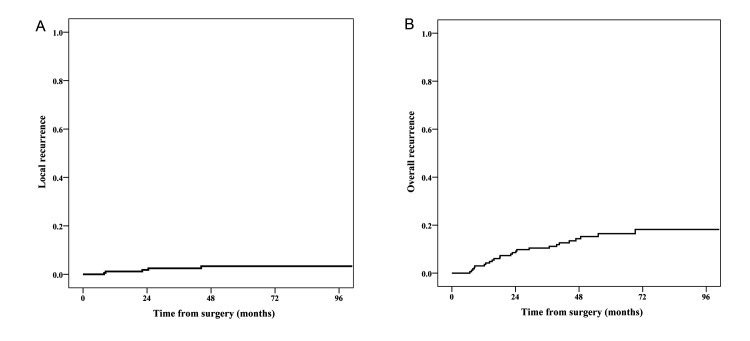
The median follow-up was 55.4 months (range, 11.7 to 100.9 months). Local recurrence was demonstrated during follow-up in 5 patients; two of them also had distant metastases detected at the same time. The actuarial overall 3- and 5-year local recurrence rates were 2.5% and 3.4%, respectively (Fig. 1A). Twenty-two patients developed distant metastases, mostly lung metastases or liver metastases (Table 2). The actuarial 3- and 5-year overall recurrence rates were 10.5% and 16.5%, respectively (Fig. 1B). In total, 16 patients died because of recurrent disease; 1 patient died from other reason. 3- and 5-year DSS were 95.6% and 90.2%, respectively.
Figure 1. Local and overall recurrence rates in patients with low-risk locally advanced rectal cancer.
(A) Cumulative local recurrence rate. (B) Cumulative overall recurrence rate.
Table 2. Survival and recurrence.
| Outcome | |
|---|---|
| Follow-up, months | |
| Median | 55.4 |
| Range | 11.7~100.9 |
| Status, no. (%) | |
| Recurrence | 25 (15.1) |
| No evidence detected | 141 (84.9) |
| 3-year RFS, % | 89.5 |
| 5-year RFS, % | 83.5 |
| Recurrence site, no. | |
| Local recurrence | 5 |
| Distant metastases | 22 |
| Lung | 11 |
| Liver | 6 |
| Brain | 2 |
| Peritoneum | 1 |
| Bone | 1 |
| Lung+liver+peritoneum | 1 |
Abbreviations: RFS, relapse free survival.
Prognostic Factors
Details of these five patients who developed local recurrence were provided in Table 3. Most recurrence occurred in male patients (4/5), with number of lymph nodes retrieved ≤12 (4/5). Local recurrence and distant metastasis rates were not significantly different in gender (male vs. female), preoperative CEA levels (≤5ng/ml vs. >5mg/ml), histology (grade I vs. grade II vs. grade III), and mucin production (mucinous adenocarcinoma vs. adenocarcinoma not otherwise specified). There was also no significant difference between patients with and without adjuvant chemotherapy in local recurrence rates (P = 0.91), RFS (P = 0.88), and DSS (P = 0.78).
Table 3. Details of patients with local recurrence.
| No. | Age | Gender | Tumor dimension (cm) | Distance to anal verge (cm) | Distance from the distal margin (cm) | Preoperative CEA level (ng/ml) | Histology | Number of lymph node retrieved | Distant metastasis detected at the same time | RFS (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 72 | Male | 3.5 | 6.0 | 2 | 3.21 | Grade II | 15 | No | 7.9 |
| 2 | 74 | Male | 2 | 10.0 | 2 | 3.22 | Grade II | 3 | Yes | 24.5 |
| 3 | 73 | Male | 5.5 | 5.0 | 2 | 3.79 | Grade I | 8 | No | 8.5 |
| 4 | 41 | Female | 4 | 10.0 | 3 | 5.15 | Grade III | 2 | Yes | 44.3 |
| 5 | 75 | Male | 3 | 12.0 | 7 | 6.55 | Grade II | 12 | No | 22.2 |
Abbreviations: CEA, serum carcinoembryonic antigen; RFS, relapse free survival.
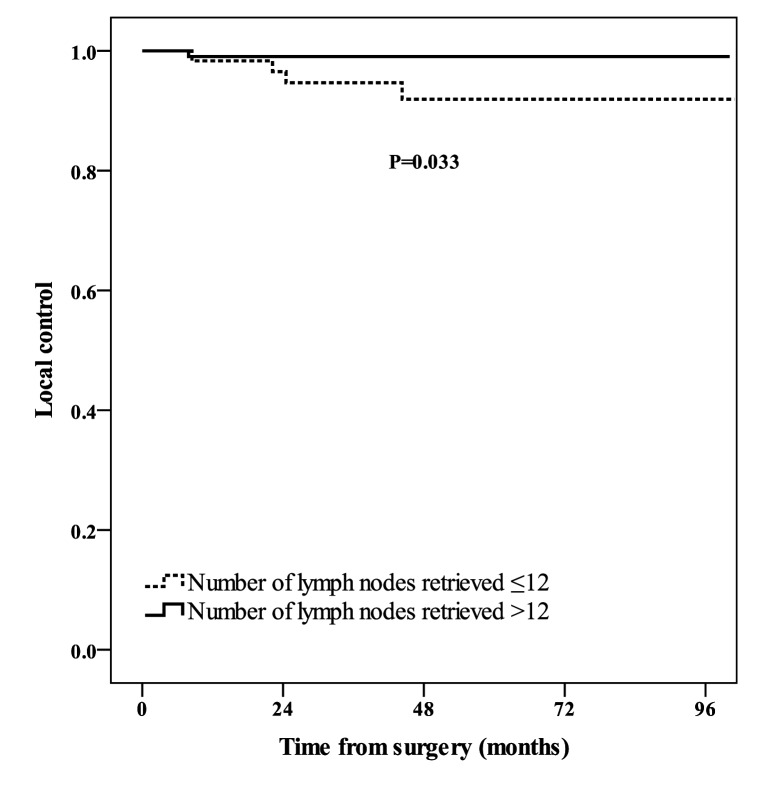
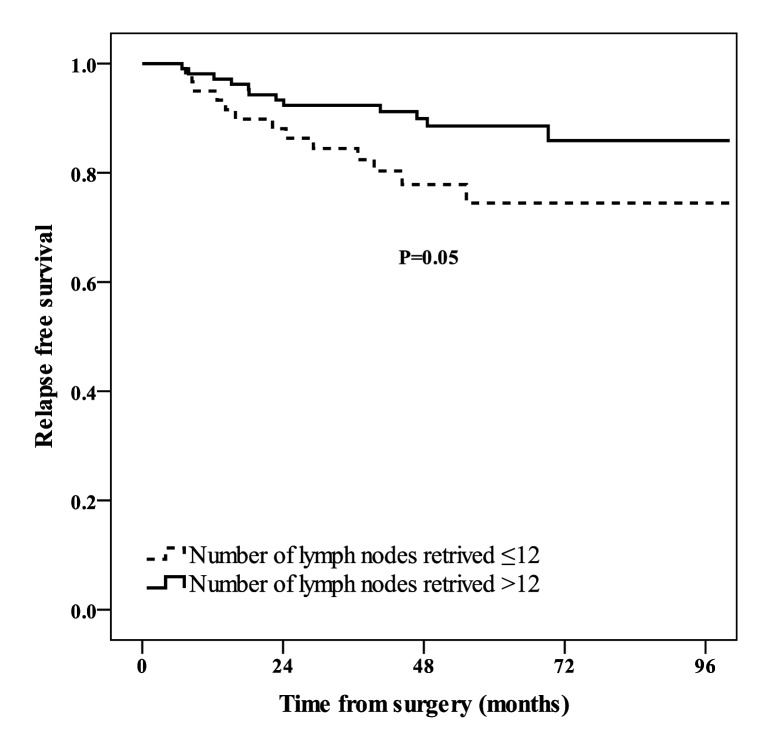
Inadequate sampling of lymph nodes (≤12) was associated with higher local recurrence rate (P = 0.03, Fig. 2). The relationship between number of lymph nodes retrieved and RFS was marginally significant (P = 0.05, Fig. 3). The 3-year RFS were 84.5% (95% CI: 79.7–89.3) and 92.4% (95% CI: 89.8–95.0) for patients with ≤12 and >12 lymph node sampling, respectively.
Figure 2. Local control of patients with number of lymph nodes retrieved≤12 (dashed line), and number of lymph nodes retrieved>12 (solid line).
Figure 3. Relapse free survival of patients with number of lymph nodes retrieved≤12 (dashed line), and number of lymph nodes retrieved>12 (solid line).
Discussion
In the current study, we identify a group of locally advanced rectal cancer with low risk of local recurrence by tumor location, preoperative MRI assessment of CRM, and TNM staging. For T3N0 rectal cancer with tumor located 5 cm above the anal verge, and without CRM involvement, the local recurrence rate is only 3.4%, which might be low enough to preclude these patients from neoadjuvant CRT.
Local recurrence was once one of the most common types of treatment failure in locally advanced rectal cancer before the standardization of TME surgery [14] and application of neoadjuvant CRT [15]. Although neoadjuvant CRT significantly decreased the risk of local recurrence in locally advanced rectal cancer, the benefit in local control could not be translated into survival benefit [10,16]. Meanwhile, the absolute decrease of local recurrence is moderate, raising the question whether we are over-treating these patients. In a Dutch trial [17], 10-year local recurrence was reduced from 11% to 5% by preoperative short-term radiotherapy. In other words, only 6% of patients benefited from neoadjuvant radiotherapy, meaning that the receipt of radiotherapy by all patients would have resulted in over treatment in 94% of patients. Meanwhile, radiotherapy or CRT inevitably brings short-term and long-term side effects, including hematologic effects, dermatologic effects, strictures at anastomotic site [15], late small bowel obstruction [18], and a substantial increase in bowel frequency and incontinence [19], which further questions the unselective application of neoadjuvant CRT to all locally advanced rectal cancer. As a result, it is critical to identify those patients with low risk of local recurrence and preclude them from the unnecessary neoadjuvant CRT.
A number of studies have demonstrated that patients underwent resection of pT3N0 rectal cancer experience a low rate of local failure after surgery alone. In a group of 108 T3N0 rectal cancer patients treated by TME without adjuvant therapy, Picon et al reported that the 5-year local recurrence rate was 8% [5]. Nissan et al reported a local recurrence rate of 4.1% in a group of patients with pT3N0 treated by radical surgery alone [4]. Similar result was also obtained by Enker showing a local failure of 4% [3]. These studies suggested that neoadjuvant CRT may be excessive for patients with T3N0 rectal cancer. However, not all patients with T3N0 can be spared from neoadjuvant CRT. Location of tumor and CRM status are also closely associated with risk of local recurrence. As reflected by the ESMO guideline for treatment of rectal cancer, a flexible strategy on application of neoadjuvant radiotherapy/CRT is recommended basing on not only clinical staging, but also location of tumor and risk of CRM involvement.
In current study, patients with lower rectal cancer (<5cm from anal verge) were excluded from the study for several concerns. First of all, tumor location is an important indicator for neoadjuvant CRT. It is well accepted that lower locating tumor is associated with higher risk of local recurrence [6]. Meanwhile, tumor involving anterior wall in lower rectum is also linked with higher local recurrence. As a result, neoadjuvant radiotherapy/CRT is recommended even when the staging is only T2 regardless of N staging if the tumor involves anterior wall in low rectum in ESMO guideline for the treatment of rectal cancer [9]. Consequently, the treatment strategy for lower rectal cancer is more complicated and should not be treated equally as the mid and upper rectal cancer. Second, previous studies [20, 21] showed that neoadjuvant CRT may facilitate sphincter-sparing surgery for lower rectal cancer. As a result, sphincter-sparing effect brought by neoadjuvant CRT should also be taken into consideration.
Similarly, pre-operative CRM (or mesorectal fascia) involvement is another critical factor that should be taken into consideration when identifying patients with low risk of recurrence. CRM is a well-established predictor of local recurrence [8]. Studies showed that CRM can be predicted by MRI with high accuracy and consistency [22, 23], allowing preoperative identification of patients at risk of recurrence who will benefit from neoadjuvant CRT, or patients at low risk of recurrence who might be spared from neoadjuvant CRT. In a study of 152 patients with rectal cancer clinically staged as T3 or T2N+, CRM at preoperative staging was the only independent preoperative factor that predicted a higher risk for local recurrence. The 5-year local recurrence rate for patients with a free preoperative CRM was 5.4% [2]. The most recent ESMO guideline of treatment for rectal cancer also emphasizes the sub-category of T3 tumor by MRI. As a result, sub-category of T3N0 tumor by MRI should be considered when identifying patients with low risk of recurrence.
Of note, in the current study, we also found that inadequate sampling of lymph nodes was associated with higher risk of local recurrence. The finding is consistent with previous reports which demonstrated that inadequate sampling of lymph nodes was associated with adverse outcome [24]. This could be explained by incorrect staging caused by inadequate lymph node sampling and the low quality of surgery. In this study, only number of lymph nodes retrieved correlated with local recurrence. In 106 patients with adequate lymph node sampling, there was only one case of local recurrence.
In this study, we defined a sub-group of locally advanced rectal cancer with low risk of local recurrence. To our knowledge, this is the first study to stratify the risk of local recurrence by a multi-factor model consisting of pre-operative CRM status, tumor location, and TNM staging. The relatively low incidence of local recurrence argues against the routine application of neoadjuvant CRT for this population.
The current study is subjective to several limitations. First, it is a retrospective analysis and was therefore limited by the bias inherent in this type of analysis. Second, it included patients with pathologic confirmed stage; whether patients with clinical staging of T3N0 have the same outcome depends on the accuracy of preoperative staging.
Conclusion
In conclusion, our data suggest that local recurrence rate after TME for upper and middle T3N0 rectal cancer with preoperative CRM>1mm is low and surgery alone may be enough for this group of patients. Large prospective randomized controlled trials should be performed to further investigate this problem.
Acknowledgments
The authors are indebted to all the surgeons that have performed the rectal cancer surgery in the department of Colorectal Surgery at Sun Yat-sen University Cancer Center. They are also grateful to all the pathologists who examined the specimens.
Data Availability
Data are available from the Sun Yat-Sun University Cancer Center Institutional Data Access / Ethics Committee for researchers who meet the criteria for access to confidential data. Contact details at the Sun Yat-Sun University Cancer Center Institutional Data Access / Ethics Committee: 651, Dongfeng Road east, Guangzhou, 510060. Tel: 8620-87343135; Email: llwyh@sysucc.org.cn. There is an ethical / legal restriction on the data set that prevents us from depositing it publicly.
Funding Statement
The authors received no specific funding for this work.
References
- 1. Engstrom PF, Arnoletti JP, Benson AB 3rd, Chen YJ, Choti MA, et al. (2009) National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: rectal cancer. J NatlComprCanc Netw 7:838–881. [DOI] [PubMed] [Google Scholar]
- 2. Frasson M, Garcia-Granero E, Roda D, Flor-Lorente B, Roselló S, et al. (2011) Preoperative chemoradiation may not always be needed for patients with T3 and T2N+ rectal cancer. Cancer 117:3118–3125. 10.1002/cncr.25866 [DOI] [PubMed] [Google Scholar]
- 3. Enker WE, Thaler HT, Cranor ML, Polyak T (1995) Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg 181:335–346. [PubMed] [Google Scholar]
- 4. Nissan A, Stojadinovic A, Shia J, Hoos A, Guillem JG, et al. (2006) Predictors of recurrence in patients with T2 and early T3, N0 adenocarcinoma of the rectum treated by surgery alone. J Clin Oncol 24:4078–4084. [DOI] [PubMed] [Google Scholar]
- 5. Picon AI, Moore HG, Sternberg SS, Minsky BD, Paty PB, et al. (2003) Prognostic significance of depth of gross or microscopic perirectal fat invasion in T3 N0 M0 rectal cancers following sharp mesorectal excision and no adjuvant therapy. Int J Colorectal Dis 18:487–492. [DOI] [PubMed] [Google Scholar]
- 6. McDermott FT, Hughes ES, Pihl E, Johnson WR, Price AB (1985) Local recurrence after potentially curative resection for rectal cancer in a series of 1008 patients. Br J Surg 72:34–37. [DOI] [PubMed] [Google Scholar]
- 7. Wibe A, Rendedal PR, Svensson E, Norstein J, Eide TJ, et al. (2002) Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cance 89: 327–334. [DOI] [PubMed] [Google Scholar]
- 8. Nagtegaal ID, Quirke P (2008) What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 26:303–312. 10.1200/JCO.2007.12.7027 [DOI] [PubMed] [Google Scholar]
- 9. Glimelius B, Tiret E, Cervantes A, Arnold D, ESMO Guidelines Working Group (2013) Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24 Suppl 6:vi81–88. 10.1093/annonc/mdt240 [DOI] [PubMed] [Google Scholar]
- 10. De Caluwé L, Van Nieuwenhove Y, Ceelen WP (2013) Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev 2:CD006041 10.1002/14651858.CD006041.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, et al. (2009) Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 373:811–820. 10.1016/S0140-6736(09)60484-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferenschild FT, Dawson I, de Graaf EJ, de Wilt JH, Tetteroo GW (2009) Preoperative radiotherapy has no value for patients with T2–3, N0 adenocarcinomas of the rectum. Dig Surg 26:291–296. 10.1159/000227771 [DOI] [PubMed] [Google Scholar]
- 13. Kim SH, Lee JM, Park HS, Eun HW, Han JK, et al. (2009) Accuracy of MRI for predicting the circumferential resection margin, mesorectal fascia invasion, and tumor response to neoadjuvantchemoradiotherapy for locally advanced rectal cancer. J MagnReson Imaging 29:1093–1101. 10.1002/jmri.21742 [DOI] [PubMed] [Google Scholar]
- 14. Heald RJ, Ryall RD (1986) Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1: 1479–1482. [DOI] [PubMed] [Google Scholar]
- 15. Sauer R, Becker H, Hohenberger W (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med.351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 16. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, et al. (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355:1114–1123. [DOI] [PubMed] [Google Scholar]
- 17. Van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, et al. (2011) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 12:575–582. 10.1016/S1470-2045(11)70097-3 [DOI] [PubMed] [Google Scholar]
- 18. Birgisson H, Påhlman L, Gunnarsson U, Glimelius B (2008) Late gastrointestinal disorders after rectal cancer surgery with and without preoperative radiation therapy. Br J Surg 95:206–213. [DOI] [PubMed] [Google Scholar]
- 19. Dahlberg M, Glimelius B, Graf W, Pahlman L (1998) Preoperative irradiation affects functional results after surgery for rectal cancer: results from a randomized study. Dis Colon Rectum 41: 543–549. [DOI] [PubMed] [Google Scholar]
- 20. Baik SH, Kim NK, Lee KY, Sohn SK, Cho CH (2008) Analysis of anal sphincter preservation rate according to tumor level and neoadjuvantchemoradiotherapy in rectal cancer patients. J Gastrointest Surg 12:176–182. [DOI] [PubMed] [Google Scholar]
- 21. Kim DW, Lim SB, Kim DY, Kim TH, Jung KH, et al. (2006) Pre-operative chemo-radiotherapy improves the sphincter preservation rate in patients with rectal cancer located within 3 cm of the anal verge. Eur J Surg Oncol 32:162–167. [DOI] [PubMed] [Google Scholar]
- 22. Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van Boven H, et al. (2001) Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet 357:497–504. [DOI] [PubMed] [Google Scholar]
- 23. Al-Sukhni E, Milot L, Fruitman M, Beyene J, Victor JC, et al. (2012) Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol 19:2212–2223. 10.1245/s10434-011-2210-5 [DOI] [PubMed] [Google Scholar]
- 24. Sarli L, Bader G, Iusco D, Salvemini C, Mauro DD, et al. (2005) Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer 41:272–279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Sun Yat-Sun University Cancer Center Institutional Data Access / Ethics Committee for researchers who meet the criteria for access to confidential data. Contact details at the Sun Yat-Sun University Cancer Center Institutional Data Access / Ethics Committee: 651, Dongfeng Road east, Guangzhou, 510060. Tel: 8620-87343135; Email: llwyh@sysucc.org.cn. There is an ethical / legal restriction on the data set that prevents us from depositing it publicly.