Abstract
Aims
There are limited and contradictory data on the effects of CRT with implantable cardioverter defibrillator (CRT-D) on mortality as compared with CRT with pacemaker (CRT-P).
Methods and results
We evaluated the long-term outcome of patients implanted with a CRT-D or CRT-P device in our high-volume single-centre experience. Data on all-cause mortality were derived from clinic visits and the Hungarian National Healthcare Fund Death Registry. Kaplan–Meier survival analyses and multivariate Cox regression models were used to evaluate all-cause mortality in patients with CRT-D vs. CRT-P, stratified by the aetiology of cardiomyopathy. From 2000 to 2011, 1122 CRT devices, 693 CRT-P (LVEF 28.2 ± 7.4%) and 429 CRT-D (LVEF 27.6 ± 6.4%), were implanted at our centre. During the median follow-up of 28 months, 379 patients died from any cause, 250 patients (36%) with an implanted CRT-P and 129 patients (30%) with an implanted CRT-D. There was no evidence of mortality benefit in patients implanted with a CRT-D compared with a CRT-P in the total cohort [hazard ratio (HR) 0.98, 95% confidence interval (CI) 0.73–1.32, P = 0.884]. In patients with ischaemic cardiomyopathy, CRT-D treatment was associated with a significant 30% risk reduction in all-cause mortality compared with an implanted CRT-P (HR 0.70, 95% CI 0.51–0.97, P = 0.03). In non-ischaemic patients, there was no mortality benefit of CRT-D over CRT-P (HR 0.98, 95% CI 0.73–1.32, P = 0.894, interaction P-value = 0.15).
Conclusions
In heart failure patients with ischaemic cardiomyopathy, CRT-D was associated with a mortality benefit compared with CRT-P, but no benefit of CRT-D over CRT-P in mortality was observed in non-ischaemic cardiomyopathy.
Keywords: Heart failure, Cardiac resynchronization therapy, Implantable cardioverter defibrillator, Mortality
Introduction
Cardiac resynchronization therapy with or without a defibrillator (CRT-D vs. CRT-P) has been shown to reduce morbidity and mortality in selected patients with heart failure and severely reduced LV function, as demonstrated in several randomized clinical trials.1–4
However, the decision of whether to implant a CRT-D or a CRT-P device has long been a controversial issue. There have been no randomized trials adequately powered that were directly comparing clinical outcome between patients implanted with a CRT-D vs. a CRT-P.
The Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial investigated CRT-D implantation vs. optimal medical therapy and CRT-P implantation vs. optimal medical therapy alone, but the trial did not evaluate the efficacy of CRT-D over CRT-P and it was not designed to answer this question.4 Several meta-analyses comparing the efficacy of CRT-D over CRT-P in patients with a primary indication for an implantable cardioverter defibrillator (ICD) have failed to show an incremental benefit of adding the defibrillator option to CRT.5–7 In addition, CRT-D devices have a higher cost and a lower cost–benefit ratio,8 and the complexity of the defibrillator leads in CRT-D systems may result in a higher risk of lead-related complications.8
Currently there is no consensus regarding the optimal patient selection for CRT-P therapy alone. Due to financial constraints, in many emerging countries physicians implant CRT-P devices in as many as 50% of their patients who would otherwise qualify for implantation of a CRT-D device by contemporary guidelines.9 Analyses identifying patient subgroups who might benefit from the implantation of a CRT-P alone are lacking and warranted.
In this high-volume single-centre CRT registry, we aimed to evaluate the effects of CRT-D vs. CRT-P on all-cause mortality in the total patient cohort and stratified by ischaemic aetiology of cardiomyopathy, as a pre-specified analysis. We hypothesized that CRT-D is associated with more pronounced mortality benefit in patients with an ischaemic aetiology of cardiomyopathy compared with CRT-P due to the reduction in sudden cardiac death (SCD).
Methods
Patient population
Between June 2000 and April 2011, a total of 1122 consecutive patients underwent CRT device implantation at the Semmelweis University Heart and Vascular Center, Budapest, Hungary. Patients undergoing CRT implantation had NYHA class II, III, or IV heart failure symptoms, QRS ≥120 ms, LVEF ≤35%, and they were on optimal medical therapy including beta-blocker, ACE inhibitor, or ARB therapy, diuretics, and an aldosterone antagonist, unless contraindicated or not tolerated by the patient.10,11 Medical therapy was optimized in all patients according to current guidelines.12 All patients provided written informed consent before the procedure.
Pre-implant assessment
Baseline clinical characteristics were recorded prior to CRT implantation. Clinical evaluation included NYHA functional class and quality of life assessment, the latter using EuroQol (EQ-5D) quality of life questionnaires.13 Two-dimensional transthoracic echocardiography was performed before CRT implantation and during follow-up using commercially available systems (Toshiba Aplio, Toshiba Medical Systems Co., Ltd, Tokyo, Japan; and Philips iE33, Andover, MA, USA). Patients had echocardiographic evaluation in the left lateral decubitus position under resting conditions. LV end-diastolic and end-systolic diameters (LVEDD and LVESD) were measured according to standard methods.14 LVEF was calculated from the apical four-chamber view images, using the Simpson disk method. Measurements were performed by the physician acquiring the echo images. Additionally, diagnostic coronary angiography and coronary artery revascularization was performed in all of our patients to exclude the need for coronary revascularization and to define the aetiology of cardiomyopathy.
Device implantation
Implantation of a CRT-D vs. a CRT-P device was left to the physician's discretion. Implantation of CRT devices was performed using the transvenous, epicardial, or transseptal approach. In patients in sinus rhythm or those with paroxysmal AF, a right atrial lead and a right ventricular lead were implanted, while patients in permanent AF received only right and left ventricular leads. During the implantation procedure, after cannulating the coronary sinus, a balloon catheter was used to perform coronary sinus venography in order to identify target veins for the LV lead. LV lead implant into the lateral or postero-lateral branch was preferred. The right ventricular lead was preferably implanted in the right septal position. LV pacing, sensing, and impedance values were measured. Phrenic nerve stimulation of the LV lead was tested in a supine position with 10 V at 2 ms pacing in the left ventricle.
In patients with intraoperative LV lead dislodgement or phrenic nerve stimulation, repositioning and stabilization of the LV lead with a coronary stent implantation was performed using the technique developed at our centre, as previously reported.15
Commercially available LV leads and CRT devices were used. If the patient received a CRT-D device, defibrillation threshold testing was performed at implantation in order to achieve a safety margin of at least 10 J.
Post-implant assessment
All patients were scheduled for an outpatient visit 1 month after device implantation and every 6 months thereafter. Clinical status assessment and device follow-up were performed at each follow-up visit and at any meaningful clinical event.
Study endpoints
The primary endpoint of the present analysis was all-cause mortality. The secondary endpoint was improvement in LVEEF as assessed by echocardiography at the last available follow-up, compared with the baseline LVEF. Data on mortality were collected from medical records and using the Hungarian National Health Fund Death Registry.
Statistical analysis
Continuous variables are expressed as the mean ± SD. Categorical data are summarized as frequencies and percentages. Baseline clinical characteristics were compared between the subgroups, stratified by CRT-D or CRT-P implant using non-parametric Wilcoxon test for continuous variables and the χ2 test for dichotomous variables.
Improvement in LVEF after device implantation, stratified by aetiology of cardiomyopathy, was compared using non-parametric Wilcoxon test, as appropriate.
The cumulative probability of survival was determined according to the Kaplan–Meier method in CRT-D and CRT-P patients, with comparisons of cumulative event rates by the log-rank test, and in the subgroups of patients with ischaemic and non-ischaemic cardiomyopathy.
Multivariate Cox proportional hazards regression analysis was used to evaluate the effect of implanted CRT-D or CRT-P device on the risk of mortality after adjustment for relevant clinical covariates that were found to be imbalanced at baseline. Interaction P-values between ischaemic and non-ischaemic patients were computed and reported to evaluate the effect of CRT-D vs. CRT-P on mortality in these subgroups.
Adjusted hazards ratios (HRs) with their 95% confidence intervals (CIs) are reported. A P-value <0.05 was considered statistically significant. Analyses were carried out with SAS software (version 9.3, SAS institute, Cary, NC, USA).
Results
Baseline clinical characteristics
Baseline clinical characteristics of patients who underwent CRT-D vs. CRT-P implantation are listed in Table 1. During the 10-year time period, 693 CRT-P (62%) and 429 CRT-D (38%) devices were implanted. Mean patient age was 65 ± 11 years and 262 (23%) of them were women. We found a lower proportion of CRT-D implantation vs. CRT-P in the early years (Supplementary material online, Figure S1).
Table 1.
Baseline clinical characteristics of patients who received cardiac resynchronization therapy with implantable cardioverter defibrillator and cardiac resynchronization therapy with pacemaker
| Clinical parameters | CRT-D patients (n = 429) | CRT-P patients (n = 693) |
|---|---|---|
| Age at enrolment (years) | 63.9 ± 10.9 | 66.3 ± 10.5* |
| Female | 68 (16) | 194 (29)* |
| Ischaemic aetiology | 220 (51) | 235 (34)* |
| Secondary prevention | 242 (56) | 70 (10)* |
| QRS (ms) | 158.2 ± 27.1 | 165.5 ± 27.8* |
| Diabetes mellitus | 134 (31) | 241 (35) |
| Hypertension | 277 (65) | 427 (62) |
| Prior MI | 230 (54) | 227 (33)* |
| Prior PCI | 124 (29) | 132 (19)* |
| Prior CABG | 88 (21) | 66 (10)* |
| Paroxysmal atrial fibrillation | 69 (16) | 94 (14) |
| Permanent atrial fibrillation | 96 (22) | 192 (28)* |
| Creatinine (µmol/L) | 114.2 ± 43.6 | 117.1 ± 53.3 |
| Urea (mM/L) | 9.8 ± 5.1 | 10.3 ± 6.4 |
| Medications | ||
| Beta-blockers | 376 (88) | 582 (84) |
| ACE inhibitor/ARB | 367 (86) | 583 (84) |
| Diuretics | 328 (77) | 522 (75) |
| Aldosterone antagonist | 259 (61) | 368 (53)* |
| Amiodarone | 180 (42) | 139 (20)* |
| Echocardiography | ||
| LVEF, % | 27.6 ± 6.4 | 28.2 ± 7.4 |
| LVEDD, mm | 65.5 ± 9.8 | 64.2 ± 9.8 |
| LVESD, mm | 55.0 ± 10.1 | 53.6 ± 10.5 |
Values are given as a percentage of patients or mean ± SD.
CABG, coronary artery bypass graft; CRT-D, cardiac resynchronization therapy with implantable cardioverter defibrillator; CRT-P, cardiac resynchronization therapy with pacemaker; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; MI, myocardial infarction.
P < 0.05 for comparison between CRT-P and CRT-D patients.
Patients with an implanted CRT-P were significantly older, were more often females, and were less likely to have ischaemic cardiomyopathy as compared with CRT-D patients. Accordingly, prior myocardial infarction (MI), prior PCI, and prior coronary artery bypass graft (CABG) were less prevalent in CRT-P patients. Patients with CRT-P more often had permanent AF and were more likely to have wider QRS complexes when compared with CRT-D patients (165.5 ± 27.8 ms vs. 158.2 ± 27.1 ms, P < 0.001).
Renal function at baseline was similar in both groups. CRT-D patients were more often prescribed aldosterone antagonists and amiodarone than those with an implanted CRT-P. Baseline echocardiographic parameters including LVEF, LVEDD, and LVESD were similar at baseline.
Device implantation
The CRT implantation procedure was performed using either a transvenous (n = 1094, 97.5%), an epicardial (n = 17, 1.5%), or a transseptal (n = 11, 1%) approach. LV leads were implanted in the lateral or postero-lateral side branch of the coronary sinus in 630 (91%) CRT-P patients and in 395 (91%) CRT-D patients, and in the anterior position in 48 (7%) CRT-P patients and 21 (5%) CRT-D patients (P-value >0.05 for the difference). Epicardial LV lead placement was performed in 10 CRT-P and 7 CRT-D patients, and the transseptal approach was used in 5 CRT-P and 6 CRT-D patients after an unsuccessful transvenous LV lead implantation.
During the implantation, LV pacing threshold, sensing parameters, and LV impedance values were determined, and all pacing outputs were programmed to achieve adequate pacing safety margins. In patients with intraoperative LV lead dislodgement or phrenic nerve stimulation, repositioning and stabilization of the coronary sinus lead was performed using coronary stent implantation.
Echocardiographic response and quality of life during follow-up in patients with cardiac resynchronization therapy with an implantable cardioverter defibrillator vs. with a pacemaker, stratified by aetiology of cardiomyopathy
Implantation of either a CRT-D or a CRT-P device was associated with significant, similar improvement in LVEF (CRT-D 6.33 ± 9.25% vs. CRT-P 6.85 ± 10.3%; P = 0.49) (Figure 1). Improvement in LVEF was similar in non-ischaemic patients with an implanted CRT-P or a CRT-D and in ischaemic patients with an implanted CRT-P or a CRT-D. However, non-ischaemic patients derived a significantly greater improvement in LVEF as compared with ischaemic patients (P < 0.001) (Figure 1).
Figure 1.
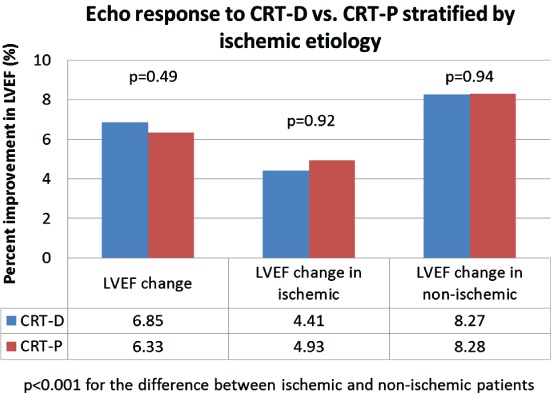
Echocardiographic response to cardiac resynchronization therapy with implantable cardioverter defibrillator (CRT-D) vs. cardiac resynchronization therapy with pacemaker (CRT-P), stratified by ischaemic aetiology.
Patients had similar improvement in quality of life with an implanted CRT-D vs. CRT-P in the total patient cohort (CRT-D, 0.20 ± 0.29 vs. CRT-P, 0.24 ± 0.29, P = 0.21), in ischaemic cardiomyopathy patients (CRT-D, 0.22 ± 0.29 vs. CRT-P, 0.25 ± 0.33, P = 0.67), and in non-ischaemic cardiomyopathy patients (CRT-D. 0.17 ± 0.28 vs. CRT-P. 0.23 ± 0.31, p = 0.14).
The risk of all-cause mortality by aetiology of cardiomyopathy
Patients with ischaemic cardiomyopathy had a significantly higher risk of all-cause mortality than those with non-ischaemic cardiomyopathy (Figure 2A), with a 5-year 52% cumulative probability of death in patients with ischaemic cardiomyopathy compared with 40% cumulative probability of death in patients with non-ischaemic cardiomyopathy (P = 0.003) that translated to an overall 40% increase in the risk of death model (P = 0.002).
Figure 2.
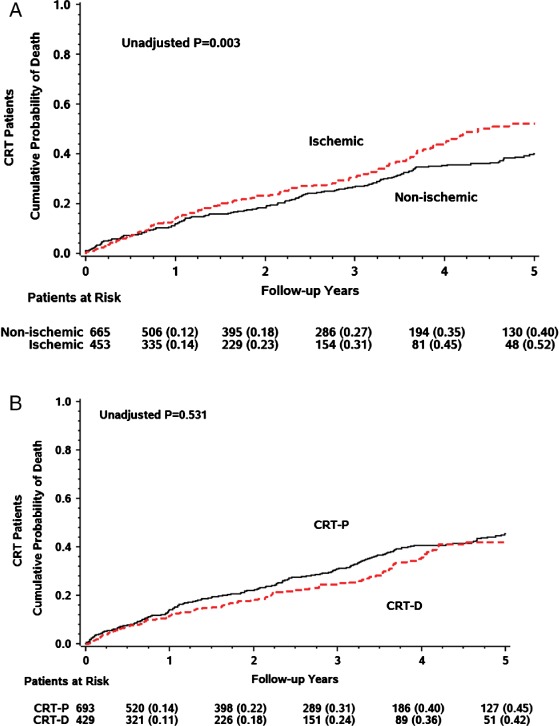
Cumulative probability of all-cause mortality (A) in ischaemic vs. non-ischaemic cardiomyopathy patients and (B) in patients with cardiac resynchronization therapy with implantable cardioverter defibrillator (CRT-D) vs. cardiac resynchronization therapy with pacemaker CRT-P).
The effect of in patients with cardiac resynchronization therapy with an implantable cardioverter defibrillator vs. with a pacemaker on all-cause mortality
During the median follow-up of 28 months (interquartile range of 12–47 months), 378 (34%) patients died of any cause, 129 patients (30%) in the CRT-D arm and 249 patients (36%) in the CRT-P arm. The 5-year cumulative survival was 56% in the total patient population.
Kaplan–Meier survival analysis of the total cohort stratified by the implanted device is shown in Figure 2B. There was no significant difference in the cumulative probability of all-cause mortality between patients implanted with a CRT-D vs. a CRT-P device, with a 5-year 42% cumulative probability of death in those with an implanted CRT-D and 45% in those with an implanted CRT-P (P log-rank = 0.531).
Univariate Cox analysis showed no difference in all-cause mortality between patients implanted with a CRT-D vs. CRT-P (HR 0.94, 95% CI 0.76–1.16, P = 0.540). Multivariate analysis after adjustment for ischaemic aetiology of cardiomyopathy, age at device implantation, and gender revealed consistent results. There was no mortality difference among patients implanted with a CRT-D or a CRT-P in the total patient cohort (CRT-D HR 0.98, 95% CI 0.73–1.32, P = 0.884) (Table 2).
Table 2.
The risk of all cause-mortality in patients with cardiac resynchronization therapy with implantable cardioverter defibrillator vs. cardiac resynchronization therapy with pacemaker in the total cohort and stratified by the aetiology of cardiomyopathy
| Parameter | Events/no. of patients | Hazard ratio | 95% CI | P-value |
|---|---|---|---|---|
| Total patient cohort | ||||
| Unadjusted CRT-D: CRT-P | 378 events | 0.94 | 0.76–1.16 | 0.540 |
| Adjusted CRT-D: CRT-P | 378 events | 0.98 | 0.73–1.32 | 0.884 |
| Ischaemic cardiomyopathy | ||||
| Unadjusted CRT-D: CRT-P | 162 events | 0.70 | 0.51–0.97 | 0.031 |
| Adjusted CRT-D: CRT-P | 162 events | 0.70 | 0.50–0.97 | 0.032 |
| Non-ischaemic cardiomyopathy | ||||
| Unadjusted CRT-D: CRT-P | 216 events | 1.03 | 0.77–1.39 | 0.839 |
| Adjusted CRT-D: CRT-P | 216 events | 0.98 | 0.73–1.32 | 0.894 |
Models are adjusted for age, gender, ischaemic aetiology of cardiomyopathy (main model only), and ischaemic aetiology–age–gender interaction.
Interaction P-value between ischaemic and non-ischaemic cardiomyopathy, unadjusted P = 0.099, adjusted P = 0.153.
CI, confidence interval; CRT-D, cardiac resynchronization therapy with implantable cardioverter defibrillator; CRT-P, cardiac resynchronization therapy with pacemaker.
The effect of in patients with cardiac resynchronization therapy with an implantable cardioverter defibrillator vs. with a pacemaker on all-cause mortality by ischaemic etiology of cardiomyopathy
In patients with ischaemic cardiomyopathy, CRT-D implantation was associated with a significantly lower cumulative probability of all-cause mortality as compared with CRT-P (5-year probability of 47% vs. 56%, P = 0.025) (Figure 3A).
Figure 3.
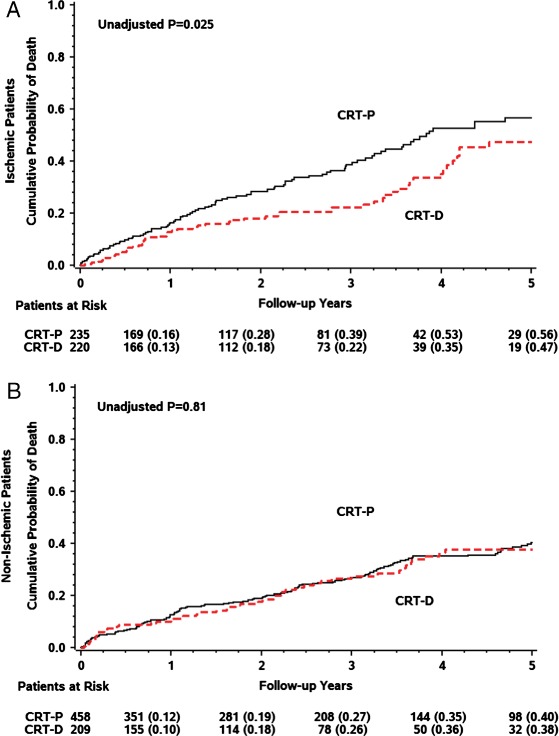
Cumulative probability of all-cause mortality in patients with cardiac resynchronization therapy with implantable cardioverter defibrillator (CRT-D) vs. cardiac resynchronization therapy with pacemaker (CRT-P) (A) in ischaemic cardiomyopathy and (B) in non-ischaemic cardiomyopathy patients.
In the univariate model, there was a 30% risk reduction in all-cause mortality in patients with ischaemic cardiomyopathy implanted with a CRT-D device when compared with patients implanted with a CRT-P (Table 3) (HR 0.70, 95% CI 0.51–0.97, P = 0.031). After adjustment for age and gender, similarly, the risk reduction in all-cause mortality was 30% with an implanted CRT-D vs. CRT-P in ischaemic patients (HR 0.70, 95% CI 0.50–0.97, P = 0.032) (Table 2).
Table 3.
Baseline clinical characteristics of patients with ischaemic aetiology of cardiomyopathy who received cardiac resynchronization therapy with implantable cardioverter defibrillator and cardiac resynchronization therapy with pacemaker
| Clinical parameters in ischaemic patients | CRT-D patients (n = 220) | CRT-P patients (n = 235) |
|---|---|---|
| Age at enrolment (years) | 65.8 ± 9.9 | 69.7 ± 8.4* |
| Female | 23 (11) | 52 (22)* |
| Secondary prevention | 124 (56) | 29(12)* |
| QRS (ms) | 159.3 ± 26.6 | 167.7 ± 30.3* |
| Diabetes mellitus | 85 (39) | 96 (41) |
| Hypertension | 156 (71) | 178 (76) |
| Prior MI | 178 (81) | 159 (68)* |
| Prior PCI | 123 (56) | 131 (56) |
| Prior CABG | 86 (39) | 65 (28)* |
| Paroxysmal atrial fibrillation | 37 (17) | 30 (13) |
| Permanent atrial fibrillation | 49 (22) | 75 (32)* |
| Creatinine (µmol/L) | 118.3 ± 39.0 | 121.4 ± 46.6 |
| Urea (mM/L) | 10.2 ± 5.3 | 10.5 ± 4.9 |
| Medications | ||
| Beta-blockers | 197 (90) | 207 (88) |
| ACE inhibitor/ARB | 191 (86) | 204 (87) |
| Diuretics | 172 (78) | 193 (82) |
| Aldosterone antagonist | 133 (60) | 126 (54) |
| Amiodarone | 96 (44) | 43 (18)* |
| Echocardiography | ||
| LVEF, % | 28.5 ± 6.7 | 29.3 ± 7.6 |
| LVEDD, mm | 64.9 ± 9.5 | 63.0 ± 9.3* |
| LVESD, mm | 54.1 ± 9.8 | 52.4 ± 10.1 |
Values are given as a percentage of patients or mean ± SD.
CABG, coronary artery bypass graft; CRT-D, cardiac resynchronization therapy with implantable cardioverter defibrillator; CRT-P, cardiac resynchronization therapy with pacemaker; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; MI, myocardial infarction.
P < 0.05 for comparison between CRT-P and CRT-D patients.
However, there was no significant difference in the cumulative probability of death from any cause in patients with non-ischaemic cardiomyopathy and an implanted CRT-D compared with those implanted with a CRT-P (Table 4) (P = 0.81) (Figure 3B). In patients with non-ischaemic cardiomyopathy, implantation of a CRT-D did not result in a beneficial reduction in mortality over what was observed with implant of a CRT-P device [unadjusted and adjusted HR 1.03 and 0.98, P = non-significant (NS) for both] (Table 2).
Table 4.
Baseline clinical characteristics of patients with non-ischaemic aetiology of cardiomyopathy who received cardiac resynchronization therapy with implantable cardioverter defibrillator and cardiac resynchronization therapy with pacemaker
| Clinical parameters in non-ischaemic patients | CRT-D patients (n = 209) | CRT-P patients (n = 458) |
|---|---|---|
| Age at enrolment (years) | 61.9 ± 11.6 | 64.6 ± 11.1* |
| Female | 164 (78) | 316 (69)* |
| Secondary prevention | 118 (56) | 6 (1)* |
| QRS (ms) | 156.9 ± 27.6 | 164.2 ± 26.2* |
| Diabetes mellitus | 49 (23) | 145 (32)* |
| Hypertension | 121 (58) | 249 (55) |
| Paroxysmal atrial fibrillation | 32 (15) | 64 (14) |
| Permanent atrial fibrillation | 47 (23) | 117 (26)* |
| Creatinine (µmol/L) | 109.1 ± 48.5 | 114.1 ± 57.4 |
| Urea (mM/L) | 9.3 ± 4.9 | 10.1 ± 7.2 |
| Medications | ||
| Beta-blockers | 179 (86) | 375 (82) |
| ACE inhibitor/ARB | 176 (84) | 379 (83) |
| Diuretics | 156 (75) | 329 (72) |
| Aldosterone antagonist | 126 (60) | 242 (53) |
| Amiodarone | 84 (40) | 96 (21)* |
| Echocardiography | ||
| LVEF, % | 26.4 ± 5.8 | 27.5 ± 7.0 |
| LVEDD, mm | 66.3 ± 10.3 | 65.0 ± 10.1 |
| LVESD, mm | 56.0 ± 10.3 | 54.5 ± 10.7 |
Values are given as a percentage of patients or mean ± SD.
CABG, coronary artery bypass graft; CRT-D, cardiac resynchronization therapy with implantable cardioverter defibrillator; CRT-P, cardiac resynchronization therapy with pacemaker; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; MI, myocardial infarction.
P < 0.05 for comparison between CRT-P and CRT-D patients.
There was a borderline significant difference in mortality reduction with CRT-D in patients with ischaemic cardiomyopathy compared with those with non-ischaemic cardiomyopathy (unadjusted interaction P-value = 0.099, adjusted interaction P-value = 0.153), suggestive of an incremental beneficial effect of CRT-D over CRT-P in ischaemic patients, but not in patients with non-ischaemic cardiomyopathy.
Discussion
We have shown in our 10-year high-volume, single-centre registry data that CRT-D is associated with a significant mortality benefit in patients with ischaemic cardiomyopathy above and beyond the benefit attained with CRT alone without defibrillator therapy. However, in patients with non-ischaemic cardiomyopathy, CRT-D implantation was not associated with mortality benefit beyond what was achieved with CRT-P alone in our registry. In the total cohort of patients, the extent of CRT-induced improvements in LVEF was similar in patients receiving either a CRT-D or CRT-P device, although the subset of patients with non-ischaemic cardiomyopathy had much greater improvements in cardiac function with CRT.
Previous randomized multicentre clinical studies evaluated the effect of CRT-D or CRT-P vs. medical therapy on all-cause mortality in patients with advanced heart failure (NYHA functional class III or IV symptoms).3 The COMPANION trial investigated CRT-D implantation vs. optimal medical therapy and CRT-P implantation vs. optimal medical therapy; however, the trial was neither designed, nor powered to compare the effect of CRT-D vs. CRT-P implantation on all-cause mortality, and therefore does not provide conclusive data for the clinician.4
The Cardiac Resynchronization Heart Failure (CARE-HF) trial compared CRT-P implantation with standard medical therapy and showed a significant mortality reduction in advanced heart failure patients with an implanted CRT-P. Furthermore, in the extended follow-up of patients enrolled in CARE-HF, the authors demonstrated that patients implanted with a CRT-P alone derived a significant reduction in the risk for SCD.16 Several other studies have shown that CRT alone reduces the risk of ventricular tachyarrhythmias and SCD due to LV reverse remodelling17 and as a result of the beneficial effects of CRT on the neurohormonal system.18 This brings into question whether improvements in cardiac function and in the neurohormonal status resulting from CRT-P alone can sufficiently lower the risk of ventricular tachyarrhythmias such that the incremental benefit from CRT with defibrillator therapy would be of limited value.
This may be the reason why several meta-analyses comparing the efficacy of CRT-D over CRT-P in patients with a primary indication for CRT have failed to show an incremental benefit of adding defibrillation therapy to CRT.5–7 The risk of ventricular arrhythmias and SCD may be sufficiently reduced with CRT-P alone. Furthermore, CRT-D devices have a significantly higher cost and their widespread use remains limited especially in emerging countries that have fixed budgets for healthcare and where healthcare utilization is based on cost–benefit analysis.8 In addition, the complex design of defibrillator leads presents additional challenges including a higher risk of lead failure in the CRT-D systems.8
Despite these great concerns, there is currently no consensus on in which patients CRT-P alone could be considered. The physician needs to estimate costs, benefit, and risks based on the individual patients. Clinicians in many countries face challenges in reimbursement of CRT devices, and expected future healthcare reforms will lead to additional scrutiny of expensive medical device therapies.9 Until clinical guidelines or consensus statements become available, our results may help clinicians identify patients in whom CRT-P alone may be sufficiently effective in reducing adverse outcomes.
Our results are novel and provocative, suggesting that CRT-D does not have an incremental benefit over CRT-P in the reduction of all-cause mortality in non-ischaemic patients. Only patients with ischaemic aetiology of cardiomyopathy showed a significant reduction in mortality with an implanted CRT-D as compared with a CRT-P. The reason for this finding may be that patients with non-ischaemic cardiomyopathy are known to be at a lower risk for ventricular tachyarrhythmias particularly in the setting of CRT-induced reverse remodelling.19 We found a significantly higher risk of all-cause mortality in patients with ischaemic cardiomyopathy compared with non-ischaemic cardiomyopathy, and this difference in the mortality risk may be due to the higher risk of life-threatening ventricular arrhythmias and SCD in ischaemic patients. This might also explain the incremental benefit of CRT-D over CRT-P in ischaemic cardiomyopathy patients. CRT-D is providing incremental benefit by reducing the risk of SCD and all-cause mortality in patients with ischaemic cardiomyopathy.
The European CRT survey evaluated baseline clinical characteristics20 and outcome21 of 2438 CRT patients with or without an ICD from 13 European countries between November 2008 and June 2009. Similarly to our study, they found that patients implanted with a CRT-D were younger, they were more often male, and they more often had ischaemic aetiology of cardiomyopathy and less often AF. However, the total cohort, as well as patients implanted with a CRT-D, in the European CRT survey was much older than the patients in our study (CRT-D patients 68 years vs. 64 years), and the proportion of ischaemic aetiology of cardiomyopathy was higher (55 % vs. 51% in our study).20 The outcome data from the European CRT survey suggested that all CRT-D patients had better survival compared with CRT-P patients during short-term, 1-year (9–15 months) follow-up.21 Differences in the clinical characteristics such as a higher percentage of ischaemic patients and an older age in the European CRT registry may explain the different findings compared with our study. However, this needs further evaluation because currently older patients are more likely to be implanted with a CRT-P device.
Another important observation of our registry data is that there was a similar improvement in LV function in patients implanted with a CRT-D vs. a CRT-P. This further underlines that since the improvement in cardiac function is the same in CRT-D and CRT-P, therefore the all-cause mortality in ischaemic and non-ischaemic patients may be equally related to heart failure-related death, but there is a difference in SCD-related death.
In our study, patients with non-ischaemic cardiomyopathy had greater improvement in LVEF than those with ischaemic cardiomyopathy. This phenomenon is well known and might be explained by the larger amount of scar tissue and lower contractile reserve in ischaemic patients that is not reversible by CRT.19 Previous studies have also shown that LVEF is related to outcome22,23 and to ventricular arrhythmias.23 Along this line, we speculate that CRT-D may provide an incremental mortality benefit over CRT-P in those who are at a higher risk for SCD at implantation (e.g. ischaemic aetiology), or in those who have less pronounced LV reverse remodelling from CRT.
It is important to note that patients implanted with a CRT-P alone did not have an increased risk of mortality compared with those with a CRT-D as shown in this analysis. The mortality risk was equal to that of those with an implanted CRT-D, with HRs and P-value close to 1, indicating a neutral effect.
Our study has several limitations. First, this is a retrospective analysis of a single-centre registry. However, we enrolled every patient implanted at our centre with a CRT device over a decade. Data collection was prospectively performed. Secondly, implantation of a CRT-D or a CRT-P device was left to the physician's discretion; it was not a randomized treatment and this may present selection bias. However, our data show ‘real-world’ clinical practice in Eastern Europe and we did not observe an increased risk in all-cause mortality in patients implanted with a CRT-P alone. Thirdly, we may be underpowered in this analysis because the interaction P-values were borderline significant; however, in the non-ischaemic group the effect was close to neutral with HRs close to 1, and the P-value was also close to 1. Previous studies suggested that an extremely large trial would be needed to have sufficient power to detect any significant differences in outcomes between CRT-D and CRT-P. However, it is unlikely that such a trial will ever be conducted, hence the importance of our study in aiding clinicians' decision-making with respect to patients in whom CRT-P alone would be sufficient. Therefore, large registry data like ours are essential to broaden clinical knowledge in this field. Further observational studies, single- and multicentre registry data, meta-analyses, and a randomized trial may be needed to confirm these findings. Fourthly, in our study, the endpoint was all-cause mortality and data on cardiac or non-cardiac mortality were not available. However, classification of cardiac and non-cardiac cause of death is often difficult and unreliable even in randomized clinical trials.
Conclusion
We demonstrated that in patients with ischaemic cardiomyopathy, there is an incremental mortality benefit from the implantation of a CRT-D device over a CRT-P device. However, in patients with non-ischaemic cardiomyopathy, there was no additional benefit in reduction of all-cause mortality in patients implanted with CRT-D as compared with CRT-P alone. Furthermore, patients with ischaemic and non-ischaemic cardiomyopathy with CRT-D or a CRT-P derived similar echocardiographic improvement. Our findings suggest that implantation of a CRT-P device might be considered in patients with non-ischaemic aetiology of cardiomyopathy who are at a low risk of SCD.
Acknowledgments
We would like to acknowledge the work of Bronislava Polonsky, programmer and statistician working at the Heart Research Follow-Up Program, University of Rochester Medical Center, Rochester, NY for the development of the Kaplan–Meier survival analysis SAS macro that we used in the current study. Furthermore, V.K. is grateful for and would like to acknowledge the support of Drs Arthur J. Moss and Wojciech Zareba from the Heart Research Follow-Up Program that was unrelated to this specific manuscript but which however helped her in gaining sufficient knowledge and experience in clinical research.
Funding
This study was supported by the Hungarian Scientific Research Fund (OTKA 105555).
Conflict of interest: L.G. has received consultant fees/honoraria from Biotronik, Medtronic, St. Jude Medical, and Johnson & Johnson; E.Z. has received consultant fees and honoraria from Boston Scientific, Innomed, Biotronik, Medtronic, and St. Jude Medical for lectures, training, and participation in clinical trials; B.M. has received consultant fees/honoraria from Biotronik, Boston Scientific, Medtronic, and St. Jude Medical, and serves on the speaker's bureau of Boehringer Ingelheim. All other authors have no conflicts to declare.
Supplementary Information
Additional Supporting Information may be found in the online version of this article:
Distribution of CRT-D and CRT-P implantation over the years
References
- 1.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA, 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 3.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. Cardiac Resynchronization-Heart Failure Study I. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 4.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 5.Lam SK, Owen A. Combined resynchronisation and implantable defibrillator therapy in left ventricular dysfunction: Bayesian network meta-analysis of randomised controlled trials. BMJ. 2007;335:925. doi: 10.1136/bmj.39343.511389.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freemantle N, Tharmanathan P, Calvert MJ, Abraham WT, Ghosh J, Cleland JG. Cardiac resynchronisation for patients with heart failure due to left ventricular systolic dysfunction – a systematic review and meta-analysis. Eur J Heart Fail. 2006;8:433–440. doi: 10.1016/j.ejheart.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 7.McAlister FA, Ezekowitz JA, Wiebe N, Rowe B, Spooner C, Crumley E, Hartling L, Klassen T, Abraham W. Systematic review: cardiac resynchronization in patients with symptomatic heart failure. Ann Intern Med. 2004;141:381–390. doi: 10.7326/0003-4819-141-5-200409070-00101. [DOI] [PubMed] [Google Scholar]
- 8.Bryant J, Brodin H, Loveman E, Payne E, Clegg A. The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review. Health Technol Assess. 2005;9:1–150. doi: 10.3310/hta9360. iii. [DOI] [PubMed] [Google Scholar]
- 9.Merkely B, Roka A, Kutyifa V, Boersma L, Leenhardt A, Lubinski A, Oto A, Proclemer A, Brugada J, Vardas PE, Wolpert C. Tracing the European course of cardiac resynchronization therapy from 2006 to 2008. Europace. 2010;12:692–701. doi: 10.1093/europace/euq041. [DOI] [PubMed] [Google Scholar]
- 10.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 29:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 11.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Riegel B, Tarkington LG, Yancy CW. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1–e62. doi: 10.1016/j.jacc.2008.02.032. 2008. [DOI] [PubMed] [Google Scholar]
- 12.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 13.Kontodimopoulos N, Argiriou M, Theakos N, Niakas D. The impact of disease severity on EQ-5D and SF-6D utility discrepancies in chronic heart failure. Eur J Health Econ. 2011;12:383–391. doi: 10.1007/s10198-010-0252-4. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Geller L, Szilagyi S, Zima E, Molnar L, Szeplaki G, Vegh EM, Osztheimer I, Merkely B. Long-term experience with coronary sinus side branch stenting to stabilize left ventricular electrode position. Heart Rhythm. 2011;8:845–850. doi: 10.1016/j.hrthm.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase] Eur Heart J. 2006;27:1928–1932. doi: 10.1093/eurheartj/ehl099. [DOI] [PubMed] [Google Scholar]
- 17.Barsheshet A, Wang PJ, Moss AJ, Solomon SD, Al-Ahmad A, McNitt S, Foster E, Huang DT, Klein HU, Zareba W, Eldar M, Goldenberg I. Reverse remodeling and the risk of ventricular tachyarrhythmias in the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy) J Am Coll Cardiol. 2011;57:2416–2423. doi: 10.1016/j.jacc.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 18.Fruhwald FM, Fahrleitner-Pammer A, Berger R, Leyva F, Freemantle N, Erdmann E, Gras D, Kappenberger L, Tavazzi L, Daubert JC, Cleland JG. Early and sustained effects of cardiac resynchronization therapy on N-terminal pro-B-type natriuretic peptide in patients with moderate to severe heart failure and cardiac dyssynchrony. Eur Heart J. 2007;28:1592–1597. doi: 10.1093/eurheartj/ehl505. [DOI] [PubMed] [Google Scholar]
- 19.Barsheshet A, Goldenberg I, Moss AJ, Eldar M, Huang DT, McNitt S, Klein HU, Hall WJ, Brown MW, Goldberger JJ, Goldstein RE, Schuger C, Zareba W, Daubert JP. Response to preventive cardiac resynchronization therapy in patients with ischaemic and nonischaemic cardiomyopathy in MADIT-CRT. Eur Heart J. 2011;32:1622–1630. doi: 10.1093/eurheartj/ehq407. [DOI] [PubMed] [Google Scholar]
- 20.Dickstein K, Bogale N, Priori S, Auricchio A, Cleland JG, Gitt A, Limbourg T, Linde C, van Veldhuisen DJ, Brugada J, Scientific C, National C. The European cardiac resynchronization therapy survey. Eur Heart J. 2009;30:2450–2460. doi: 10.1093/eurheartj/ehp359. [DOI] [PubMed] [Google Scholar]
- 21.Bogale N, Priori S, Cleland JG, Brugada J, Linde C, Auricchio A, van Veldhuisen DJ, Limbourg T, Gitt A, Gras D, Stellbrink C, Gasparini M, Metra M, Derumeaux G, Gadler F, Buga L, Dickstein K. Scientific Committee, National Coordinators, and Investigators. The European CRT Survey: 1 year (9–15 months) follow-up results. Eur J Heart Fail. 2012;14:61–73. doi: 10.1093/eurjhf/hfr158. [DOI] [PubMed] [Google Scholar]
- 22.Moss AJ. Prognosis after myocardial infarction. Am J Cardiol. 1983;52:667–669. doi: 10.1016/0002-9149(83)90394-6. [DOI] [PubMed] [Google Scholar]
- 23.Kutyifa V, Kloppe A, Zareba W, Solomon SD, McNitt S, Polonsky S, Barsheshet A, Merkely B, Lemke B, Nagy VK, Moss AJ, Goldenberg I. The influence of left ventricular ejection fraction on the effectiveness of cardiac resynchronization therapy: MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy) J Am Coll Cardiol. 2013;61:936–944. doi: 10.1016/j.jacc.2012.11.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of CRT-D and CRT-P implantation over the years


