Fig 16. Synthesis of aminoquinoline 8.
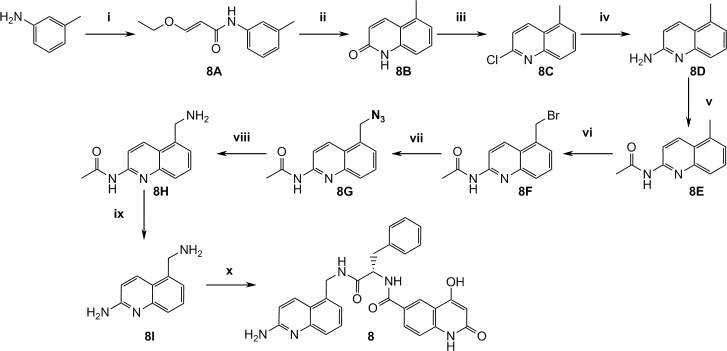
i) (E)-3-Ethoxyacryloyl chloride, THF, 50°C, 16h, ii) Conc. HCl, 40°C, 1h, iii) Neat POCl3, 80°C, 3h, iv) Xantphos, Pd2(dba)3, tBuOK, PhMe, 100°C, 1h, then 2M HCl and THF, r.t, 2h, v) AcCl, TEA, DCM, N2, 0°C, 2h, then excess MeNH2 r.t, 16h, vi) NBS, benzoyl peroxide, (trifluoromethyl)-benzene, 80°C, 16h, then reflux 2h, vii) NaN3, DMF, 80°C, 1h, viii) Pd(OH)2 on carbon, EtOH, H2, r.t, 16h, ix) 20% NaOH, 120°C, 1h, x) DMF, DIPEA, 15B, TBTU r.t, 16h.
