Introduction
Our current healthcare system is the beneficiary of the landmark successes of earlier pioneers who struggled, but persevered, to save lives. In the 19th century, two individuals stand out. Dr Louis Pasteur, a PhD basic scientist who luckily was ‘encouraged’ to conduct applied research and saved a life. Professor Paul Erhlich, a medically qualified research pathologist and winner of the Nobel Prize for antitoxin research, would create a model for successful synthetic drug development that would save thousands of lives. In my generation, it was my friend and supporter Sir James Black, Nobel laureate, who would advance the selectivity implied by receptor theory to treat patients for long periods with pathological conditions. Infectious diseases were cured within months but chronic heart disease, elevated blood pressure and gastric acid secretion were stabilized for years. Lives were saved and the practice of medicine changed to become evidence based. The key to success throughout was the creation and use of appropriate animal models.
In this article, I will focus on the essential aspects of animal models in the unanticipated development of an orphan medicine tamoxifen, used initially to treat late stage breast cancer. The results from the animal models taught the medical profession how to use tamoxifen effectively to save lives, how to detect life-threatening side effects, or provided clues about a new group of medicines that now have multiple applications in women's health. But first, what did our pioneers do and how did they do it?
A perspective on pioneers
Dr Louis Pasteur had already had a prestigious career studying crystal structure, inventing ‘pasteurization’ for milk and wine to stop spoilage and a vaccine to protect sheep from anthrax, when he turned his attention to the fatal disease rabies. He used a rabbit model to attenuate the rabies virus and a dog model to test the vaccine 1. His initial goal was to develop an experimental vaccine for study in animals until the fateful day the mother of 9-year-old Joseph Meister pleaded with Pasteur to save her son from a slow and painful death. He had been severely bitten by a rabid dog and death was assured. The unexpected arrival of the young Joseph Meister at that moment was critical, as Pasteur had recently revised his method to prepare attenuated rabies virus and the strategy to treat dogs to protect them from rabies. Pasteur found through his earlier experiments that passing the virus through monkeys, was not optimal and he selected passage through rabbits and collected the infected spinal cords. He fixed them by drying inside flasks protected from moisture. Two weeks of drying reduced the extracted virus to become harmless to dogs who were now immune to rabies once inoculated with preparations of increasing virulence based on less dessication time. The young Meister was injected over a period of 11 days with a total of 13 injections of increasing rabies virulence. He escaped certain death. Following Pasteur's death and burial in the crypt of the Pasteur Institute in Paris, Joseph Meister became the faithful custodian to this medical pioneer's memory until the German occupation of Paris in 1940. It is said he chose suicide rather than surrender the keys to the crypt and the memorial to the scientist who saved his life and changed medicine.
Professor Paul Ehrlich devised the drug discovery and development system used today 2. Earlier in his career as a pathologist he was fascinated to find that organic dyes would specifically bind to bacterial and not human tissue. This gave him the clue to devise chemical therapy. Ehrlich's primary interest was vaccines and antitoxins for which he received the Nobel Prize. Ehrlich believed in the fidelity of the immune system to neutralize and destroy infectious disease. However with the expansion of European colonial interests into Africa came new challenges. It became obvious that the immune system could not kill tropical diseases such as malaria and sleeping sickness whose cause was protozoal. The immune system was overwhelmed by the sheer bulk of the infectious agent. Ehrlich stated ‘an attempt must be made to kill the parasites within the body by chemical agents. In other words chemical agents must be used when serum therapy is impossible. French scientists Alphonse Laurier (awarded the Nobel prize for the discovery of the causative agent of malaria) and Mesnel found they could transfer trypanasomes from mouse to mouse to replicate human disease. Progression of the disease could be monitored through blood tests.
Ehrlich used the model to show that dyes could be ‘parasitotropic’ in mice. Trypan red could cure infected mice. However, when Ehrlich identified the nitrogen-containing azo group in trypan red this brought him to organic arsenicals. An arsenical para-aminophenyl arsenic acid (atoxyl) was marketed already but the compound was ineffective in their model. They had discovered arsenical resistance. A fortunate series of scientific advances in microbiology in 1905 occurred with the chance observation by others, that syphilis was associated with spirochetes that occupied a position between protozoans and bacteria. This was followed by the validation of animal models by scientists in Italy in 1906. At this point Ehrlich appears to have integrated a study of syphilis and a study of resistance to trypanosomes to arsenicals into his laboratory strategic plan. The key to success for the eventual discovery of compound 606 (Salvarsan), through methodical structure activity relationship, was the recruitment of Sahachiro Hata from Tokyo to screen all the compounds in the appropriate models of human disease. Salvarsan was discovered in June 1909. Following toxicology in animals, clinical trials were conducted with the drug manufactured by the Hoechst Company in Germany. Another deadly infectious disease was cured and thousands lived.
Sir James Black (of β-adrenoceptor blocker fame) 3 worked in the laboratories of Imperial Chemical Industries (ICI) Pharmaceuticals Division, Alderley Park, near Macclesfield, Cheshire. He had left ICI by the time I was a summer student at ICI in 1967. Alderley Park was 10 miles from my home and I was then an undergraduate in the Pharmacology Department at Leeds University, keen to do research in cancer drug discovery. There was none of significance then at ICI but the cell biologist Dr Steven Carter (of cytochalasin B fame) 4 was looking at the effects of compounds on mouse cancer cells in culture. It was a start! Coincidentally, the Head of the Fertility control programme, Dr Arthur Walpole had his laboratory opposite Dr Carter's. He had just published a paper 5 on the effects of ICI 46 474 as a ‘morning after pill’ in rats – but nobody cared! We will meet ICI 46 474 (tamoxifen) later.
Although this was a prescient meeting with Dr Walpole as he would later be the examiner of my PhD on ‘failed morning after pills’ at Leeds in 1972, the critical players at the start of our tale were being assembled. I met Dr Michael Barrett (of atenolol fame) 6 whose laboratory was next to Dr Carter's at ICI. He had taken over the β-adrenoceptor blocker programme after Jim Black left. Dr Barrett was to talent spot me for a faculty position at Leeds when he became the Professor of Pharmacology in 1970.
Also at ICI in the summer of 1967, I had the privilege to meet Dr James Raventos who was studying gastric acid secretion in dogs with histamine. Jim later told me that the known antihistamines did not block histamine stimulated gastric acid secretion in the dog model. Based on Jim's pioneering studies on the regulation of accelerated cardiac function and arrhythmias in the dog model with his new β-adrenoceptor blockers, he reasoned that the ‘antihistamine anomaly’ must be because there was a second subtle histamine receptor modulating mechanism 3 – and so it was. The H2-receptor blockers were born at Smith, Kline and French and long term treatment with H2–receptor blocker ‘antacids’ was possible as was β-adrenoceptor blocker treatment for heart conditions before.
Regulations for the safety of medicines
Pasteur, Ehrlich and Black each chose not to conform to the dogma that disease and death were inevitable. Each chose to question Nature through experimental animal models. Their persistence was translated to patient care. However, success in one area of therapeutics demands regulations imposed by society on claims in other areas thereby preventing Charlatans peddling ‘cures’ that are neither evidenced based nor safe. The elected representatives of the people in our democratic society are charged with the responsibility to enact laws and regulations that ensure the safety of any new medicine. A strict protocol of appropriate animal toxicology is enforced by acts of government to prevent unanticipated injury or deaths.
It is not necessary to expand further as the concepts of safety and the documented worth of a medicine for patient care should be obvious to all. Nevertheless, a couple of examples will be given to illustrate instances when an inadequate system of protection can fail or a warning model appears to do so.
It is axiomatic that one should always err on the side of caution with safety and side effects of medicines. Thalidomide taught us that lesson so why was there no caution? The reason that the tragedy occurred was that there was no legal requirement to test for teratogenicity in the 1950s 7. The toxicology concern was first raised by observation in humans 8. Tragically, the value of thalidomide was seen to be in the control of nausea in pregnant women during the first trimester 9, exactly when limb development is occurring in the foetus. It is now known that thalidomide can stop blood vessel formation and limb formation is particularly vulnerable. Now there is rigorous teratogenesis testing of medicines to be used in women of childbearing age. It is important to note that thalidomide used in a cancer context, to treat a fatal disease, can produce improvements in multiple myeloma deployed as an anti-angiogenic agent.
The thalidomide tragedy and introduction of teratogenic testing is why women taking the anti-oestrogen tamoxifen during their childbearing years to treat breast cancer, must use barrier contraception to prevent pregnancy. However, there was an apparent anomaly in the toxicology testing of tamoxifen when it transitioned from cancer therapy with a requirement for only liberal toxicity testing for a fatal disease, to a chemopreventive in disease-free women only at risk for breast cancer. This toxicological surprise in rats given tamoxifen for years was hepatocellular carcinoma that was first reported 10 nearly 20 years after tamoxifen had been used by perhaps a million women worldwide.
Tamoxifen was discovered to be a rat liver carcinogen at high doses given for a lifetime 10 but increases in human hepatocellular carcinoma were not noted either in the 1990s 11,12 when the toxicological issue was raised initially or indeed now 13. Millions of women have benefited from tamoxifen with its long term use. However, tamoxifen would not have been knowingly developed by any company had the toxicological knowledge been available at the beginning of the tamoxifen tale in 1973 14. Without the success of tamoxifen as a lifesaving medicine there were no agents waiting as the ‘first reserve’ anti-oestrogen – nobody cared. Without the success of tamoxifen, there would have been no financial incentive to develop aromatase inhibitors 15 and there would have been no selective oestrogen receptor modulators (SERMs) 16,17. So this would seem to argue against animal testing? Certainly not. The toxicological requirements for an anticancer therapy to delay a fatal disease are rightly less draconian than for the treatment of a subject with an infection or no life-threatening disease. The fact the rat liver carcinogenesis was discovered after 20 years of tamoxifen use, allowed the clinical and toxicological community also to evaluate ‘real world’ experience in women 11,12 No increase in liver cancer was noted. Scientists were able to determine that the rat is particularly susceptible with its metabolism of tamoxifen to producing a carcinogen but the human rapidly repairs DNA damage 18. The system for protecting human safety for the introduction of an unknown drug to prevent a disease worked with appropriate toxicology testing in animals. Cancer patients lived because of appropriate testing and risk management for treatment of a fatal disease.
The early years of the tamoxifen tale
Cancer therapeutics and cancer prevention are a particular challenge as we seek to destroy renegade cells that are ‘self’. Ehrlich chose to explore the development of appropriate animal models of human disease to address cancer chemical therapy (chemotherapy) at the dawn of the 20th century. In the year before he died in 1916 he declared ‘I have wasted 15 years of my life on experimental cancer research’ 19.
The banner of progress in therapeutics was picked up in the 1940s using a process of translational research i.e. first validation of an antitumour response in animal models and then a clinical trial. Sir Alexander Haddow FRS discovered 20 that high dose synthetic oestrogen treatment could produce a 30% response rate in breast cancer patients more than 5 years after their menopause 21. This was the first chemical therapy to treat any cancer successfully and was proven in clinical trials. However, high dose oestrogen treatment is a paradox as all other approaches before the Haddow breakthrough caused regression of breast cancer by endocrine ablation (oophorectomy, adrenelectomy), i.e. taking away oestrogen just as tamoxifen blocks oestrogen from stimulating tumour growth. High dose oestrogen therapy remained the treatment of choice for breast cancer after the menopause for the next 30 years until the introduction of tamoxifen (1973 UK, 1977 USA) with fewer side effects 22,23. The only randomized trial 23 of high dose oestrogen vs. tamoxifen in unselected (no oestrogen receptor (ER) selection) post-menopausal patients with metastatic breast cancer was quite small with 74 patients and 69 patients, respectively. Response rates were both about 30% and disease control was similar over a 2 year period. Only the increased side effects noted with high dose oestrogen led the authors to recommend tamoxifen 23.
It is interesting to note that all the early events in the story of breast cancer ‘chemical therapy’ are actually connected. Haddow's experimental oestrogens were synthesized by ICI 20. Walpole was specifically interested in cancer research. He tried unsuccessfully to discover why only some tumours responded to oestrogen therapy 24 and successfully developed an early ‘chemotherapy’ 25 but was directed to create a safer ‘morning after pill’. The discovery by scientists in America that synthetic oestrogens could be converted to anti-oestrogens through the skill of the medicinal chemist 26 that were also effective ‘morning after pills’ in the rat which could potentially now create another ‘blockbuster’ in the wake of the success of oral contraceptives. My connection with the anti-oestrogen research team at ICI throughout the 1970s has recently been told 27 and the clinical development of tamoxifen explained 28,29. However, tamoxifen is not about a single medicine but the pioneer in a group of medicines now called SERMs.
Forty years ago there were no SERMs, today there are five but with a sixth, lasofoxifene, approved in the European Union a few years ago. This approval has lapsed (Figure 1). The SERMs were predicted to treat multiple diseases in post-menopausal women simultaneously 26. The currently approved SERMs treat breast cancer, prevent breast cancer, prevent osteoporosis and preparations prevent menopausal symptoms including dyspareunia. The general outline of the development of the two principal SERMs, tamoxifen and raloxifene, are illustrated and explained in Figures 2 and 3 and a current view of the molecular mechanism of action illustrated for target sites in Figure 4. These stories have been explained recently in detail 30,31. However, none of the SERM story would have occurred but for the appropriate use of animal models to guide clinical trials, to understand patient safety and finally to define a new biology of oestrogen-induced apoptosis. This cascade of knowledge answered the question ‘how can oestrogen stimulate breast cancer growth (which is the basis of all successful anti-oestrogenic therapy for the past 40 years 32) but also cause apoptosis as a breast cancer therapy 22,23 ’. It is animal models that aided the understanding of ‘Haddow's paradox’ 21 that oestrogen can kill correctly prepared breast cancer cells. That knowledge and the molecular mechanism again have clinical significance.
Figure 1.
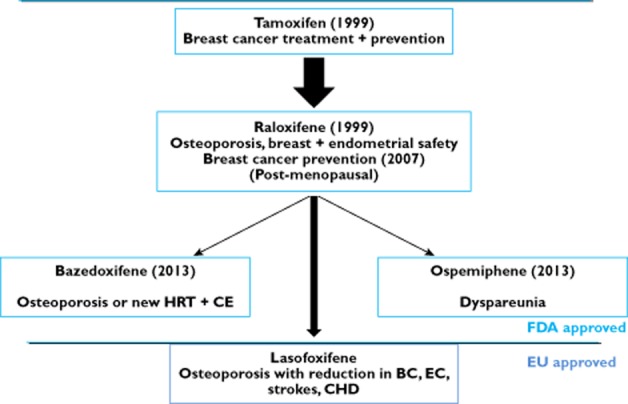
The approvals of individual selective oestrogens receptor modulators (SERMs) in the United States of America through the evaluation system of the Food and Drug Adminisration (FDA). Approvals were specifically for indications at the highest level of toxicologic safety for women without disease but as a new hormone replacement therapy with conjugated oestrogen (HRT + CE) to prevent disease i.e. chemoprevention of osteoporosis, breast cancer (BC), menopausal symptoms or dyspareunia. One SERM, lasofoxifene, was approved for use in the European Union (EU) but was never launched or marketed despite the fact that clinical trials demonstrated a reduction in breast cancer (BC), osteoporosis fracture, strokes, endometrial cancer (EC) and coronary heart disease (CHD) 92
Figure 2.
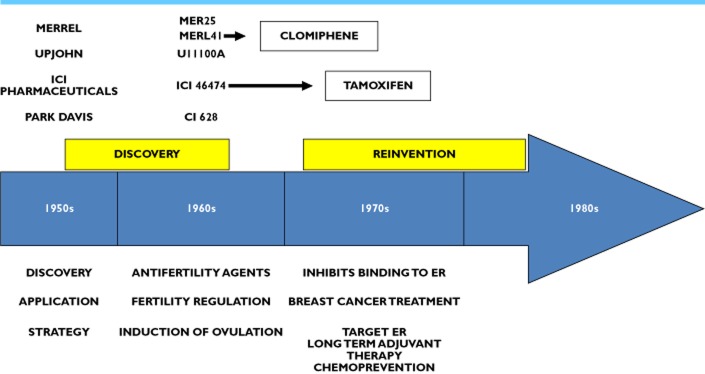
A trickle to tamoxifen (ICI 46 474). During the 1960s, a number of triphenylethylene derivatives were discovered that were excellent novel post-coital antifertility agents in rats but induced ovulation in subfertile women (clomiphene and tamoxifen) 26. Tamoxifen moved forward as a palliative treatment for metastatic breast cancer, only after being all but abandoned as a commercially viable enterprise. It was then rescued as an orphan drug in 1972 93. Laboratory models informed about possible applications as a long term adjuvant therapy or as a chemopreventive agent 27. Clinical trials demonstrated major survival advantages for women with ER positive breast cancer who received long term (>5 years) tamoxifen therapy and tamoxifen was tested and was the first medicine to be approved for the reduction of breast cancer in high risk women 93
Figure 3.
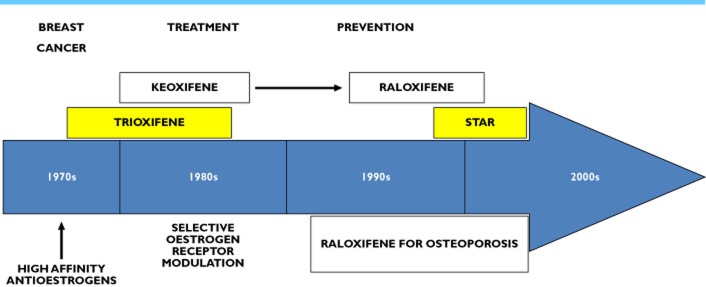
Rush to raloxifene. The success of tamoxifen for the treatment of breast cancer created potential opportunities to develop drugs to correct toxicological issues of concern i.e. the increase in endometrial cancer. Trioxifene was developed as a potential competitor for tamoxifen but failed to demonstrate either increased efficacy in the treatment of metastatic breast cancer or decrease in serious side effects. In the wake of the discovery that tamoxifen was metabolically activated to 4-hydroxytamoxifen with high binding affinity for the ER (Figure 2) 70,94 a compound LY156758 or keoxifene was developed that had high binding affinity for the ER and did not have oestrogen-like activity in the uterus 95. Keoxifene failed to become a breast cancer therapy and was abandoned in 1987. However, the discovery that keoxifene prevented bone loss and mammary cancer in rats 51,75 ultimately resulted in the resurrection of the molecule as raloxifene. The clinical testing resulted in the approval of raloxifene to treat and prevent osteoporosis in post-menopausal women in 1997 and for the reduction of the incidence in breast cancer in high risk post-menopausal women in 2006. This was the Study of Tamoxifen and Raloxifene (STAR). Unlike tamoxifen, raloxifene does not increase the incidence of endometrial cancer 78
Figure 4.
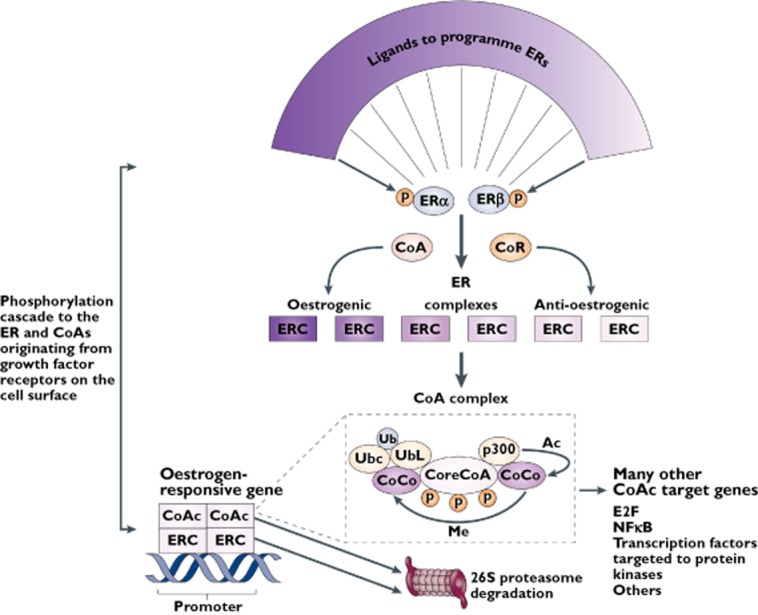
The oestrogen target tissue decision network for selective oestrogen receptor modulation. The shape of the ligands that bind to the oestrogen receptors (ERs) α and β programmes the complex to become an oestrogenic or anti-oestrogenic signal. The context of the ER complex (ERC) can influence the expression of the response through the numbers of co-repressors (CoR) or co-activators (CoA). In simple terms, a site with few CoAs or high levels of CoRs might be a dominant anti-oestrogenic site. However, the expression of oestrogenic action is not simply the binding of the receptor complex to the promoter of the oestrogen-responsive gene, but a dynamic process of CoA complex assembly and destruction. A core CoA, for example, steroid receptor coactivator protein 3 (SRC3), and the ERC are influenced by phosphorylation cascades that phosphorylate target sites on both complexes. The core CoA then assembles an activated multiprotein complex containing specific co-co-activators (CoCo) that might include p300, each of which has a specific enzymatic activity to be activated later. The CoA complex (CoAc) binds to the ERC at the oestrogen-responsive gene promoter to switch on transcription. The CoCo proteins then perform methylation (Me) or acetylation (Ac) to activate dissociation of the complex. Simultaneously, ubiquitiylation by the bound ubiquitin-conjugating enzyme (Ubc) targets ubiquitin ligase (UbL) destruction of protein members of the complex through the 26S proteasome. The ERs are also ubiquitylated and destroyed in the 26S proteasome. Therefore, a regimented cycle of assembly, activation and destruction occurs on the basis of the preprogrammed ER complex. However, the co-activator, specifically SRC3, has ubiquitous action and can further modulate or amplify the ligand-activated trigger through many modulating genes that can consolidate and increase the stimulatory response of the ERC in a tissue. Therefore, the target tissue is programmed to express a spectrum of responses between full oestrogen action and anti-oestrogen action on the basis of the shape of the ligand and the sophistication of the tissue-modulating network. NFkB, nuclear factor kB. Reprinted with permission from the Nature Publishing Group, Jordan 96
The role of appropriate animal models in breast cancer research to save lives
In 1972, just 2 months after my successful PhD examination with Dr Arthur Walpole on the topic of ‘failed morning after pills’ entitled A study of the oestrogenic and anti-oestrogenic activities of some substituted triphenylethylenes and triphenylethanes, I found myself at the Worcester Foundation for Experimental Biology in Shrewsbury, Massachusetts, USA. I discovered I was to be an independent investigator working in the ‘home of the oral contraceptive’ but I chose to explore the possibility with ICI of contributing to the development of their orphan drug ICI 46 474 (but not yet tamoxifen). During the time I was at the Worcester Foundation (1972–1974) there were only two clinical reports 22,33 of the use of tamoxifen to treat breast cancer, but these were not randomized trials, there was no correlation between tumour ER and endocrine ablation, that was to be published in 1975 34, and there was no mention of tamoxifen as it was not used in this context. A correlation between response and tumour ER was noted later 35,36. Adjuvant tamoxifen therapy and chemoprevention were not on the clinical landscape. There was much to do at the beginning to develop a rationale to advance a ‘failed morning after pill’. They funded my research proposal but how to start. I needed to train myself to find a model to evaluate and quantify the antitumour effect of ICI 46 474. No laboratory antitumour studies had been done by ICI (or anyone else) but as Ehrlich had taught an ‘appropriate animal model of human disease was necessary’ to convince the clinical cancer community to conduct clinical trials. The prowess of ICI 46 474 as an effective ‘morning after pill in rats’ would not suffice!
I found my model in Chicago at the Ben May Cancer Research Laboratories of the University of Chicago. I visited at the invitation of the Director, the late Dr Elwood V. Jensen in the spring of 1973. I learned the ‘Jensen method’ of measuring the tumour ER, an enormous improvement over my ‘Heath Robinson’ approach alone in the basement of Leeds University Old Medical School during my PhD. I learned the dimethylbenzanthracene (DMBA)-induced rat mammary carcinoma model 37 and had the good fortune to meet and talk with Professor Charles Huggins, the former director of the Ben May laboratory for Cancer Research and Nobel Laureate for his work on hormone dependent prostate cancer. This readily reproducible mammary tumour model is hormone (ovarian) dependent for growth and the tumours contained the ER 38. It was the only appropriate model. For the next decade this model would be my medium to propose targeting the ER positive tumour 39 with long term adjuvant tamoxifen therapy 40–42 or use the animal model in the first step towards chemoprevention of breast cancer 43,44. All of this would occur at the Worcester Foundation (1972–1974) and at the Department of Pharmacology of the University of Leeds (1974–1979). The next dimension in discovery for therapeutics would occur in the 1980s at the University of Wisconsin Clinical Cancer Center (Madison) (1980–1993) in the United States.
The nu/nu athymic mouse model was found to be immune deficient so human tumours could be transplanted and therapies studied to seek cures for cancer 45. Of particular interest to my new embryonic tamoxifen team in the Department of Human Oncology at the Clinical Cancer Center were the observations that the ER positive human breast cancer cell line MCF-7 46,47 inoculated into the axillary mammary fat pad was able to grow into tumours with oestrogen treatment 48,49. Furthermore, tamoxifen prevented oestrogenic-stimulated tumour growth 50. Here was the new model we needed.
Marco Gottardis was an extremely talented technician conducting animal studies in the Department of Human Oncology in the Cancer Center. He accepted my invitation to become a graduate student in my laboratory. His work and publications changed therapeutics and medical care multiple times as he expertly used carcinogen-induced rat mammary tumour models 51 and established our colony of MCF-7 tumour bearing athymic mice 52. The latter model revolutionized our understanding of acquired resistance to long term tamoxifen therapy 53 and what to do about it in the clinic 54. The athymic mouse model would provide the leads to the target site specificity of ‘non-steroidal anti-oestrogens’ 55,56. Harper & Walpole 57 had discovered the unusual species specificity to ICI 46 474. The triphenylethylene was classified as an oestrogen in the mouse vagina and this classification was confirmed by Terenius in immature micee with uterine weight tests 58. ICI 46 474 was classified as an anti-oestrogen in the rat with partial agonist uterine action 5. However the fact that ICI 46 474 (tamoxifen) acted as an anti-oestrogen to block oestrogen stimulated tumour cell growth in athymic mice 55 was a first clue that tamoxifen was tissue, not species, specific. The development of this observation in different target tissues would give the insight into a new group of medicines in women's health, the SERMs that switch on or switch off oestrogen target sites around the body 59. This is a fascinating story in molecular pharmacology as the interpretation of the two known ERs, i.e. α and β with different coregulators and receptor processing at different gene promoters, can produce agonist or antagonist action. This multifaceted decision network is summarized in Figure 4. Marco is now the Vice President and Prostate Cancer Disease Area Stronghold Leader for the Oncology Therapeutic Area at Janssen Research and Development, LLC in New York.
It would be another graduate student, Doug Wolf who would have the transplantable model of acquired resistance to tamoxifen passed to him! He would discover that after retransplantation of the tumours for years into successive generations of tamoxifen-treated athymic mice, that physiological oestrogen could make tumours melt away 60. This serendipitous discovery at Wisconsin would be developed fully 61 at the Robert H. Lurie Comprehensive Cancer Center at Northwestern University, Chicago (1993–2005) by surgical residents, medical oncology fellows or scientists: Kathy Yao, Gale England, Eun-Sook Lee, David Bentrem, Ruth O'Regan, Rita Dardes, Jennifer MacGregor, Hong Liu, Clodia Osipo, Debra Tonnetti and Joan Lewis all co-operated and achieved successes 61–67. Our tamoxifen teams have remained an essential balance of clinical and laboratory expertise to ensure we never lose sight of the goal – improving cancer care. Doug Wolf is now the Senior Director Oncology regional medical research specialist at Pfizer.
I will illustrate the translational aspects of our tamoxifen tale by our tamoxifen teams over the decades with the following examples of successful translational research outcomes.
An appropriate strategy for the adjuvant antihormone treatment of breast cancer
Laboratory model
The DMBA-induced rat mammary carcinoma model has been examined extensively by hundreds of investigators 38 but the main hypothesis to be tested in our studies was that longer treatment starting when animals had only occult disease following DMBA administration was superior to short term therapy 40–42. The secondary hypothesis to be addressed was that only ER positive disease would respond as tamoxifen and metabolites blocked the binding of [3H]-oestradiol to tumour ER 39,68–70.
Clinical translation
The overviews of clinical trials conducted every 5 years at Oxford demonstrated that only patients with an ER positive primary tumour responded to adjuvant tamoxifen and longer therapy (5 years) was superior to either 1 or 2 years of adjuvant tamoxifen 12,13. There was a 50% decrease in recurrence rates and a 30% decrease in mortality. Maybe a million lives were saved.
Tamoxifen and target site specific anticancer action
Laboratory model
Athymic mice were transplanted with an ER positive breast cancer and an ER positive endometrial cancer and treated with oestrogen to stimulate growth. Tamoxifen was administered to determine whether the anti-oestrogen controlled the growth of both breast and endometrial cancer. Breast cancer was controlled but endometrial cancers grew dramatically 56.
Clinical translation
Marco Gottardis and I presented these data prior to publication to staff at ICI Pharmaceuticals Division, Alderley Park. In 1987, I presented the results and my concerns at a medical conference organized during the celebration of the 900th anniversary of the first university in the world, the University of Bologna, Italy. As a result of my lecture, Dr Leonard Hardell wrote a letter to the Lancet 71 and I replied appealing for results from a large prospective clinical trial 72. The database from Fornander and colleagues 73 demonstrated that longer tamoxifen (5 years) caused the detection of more endometrial cancer than shorter (2 years) of adjuvant tamoxifen. The report also confirmed that the incidence of new primary breast cancers was reduced by tamoxifen but endometrial cancer incidence went up. I replied 74. Medical practice changed with new package inserts and gynaecologists became involved as part of the breast cancer patient care team. The whole process of translational research to clinical practice took 2 years and almost certainly saved lives.
The discovery of SERM action
Laboratory model
In the 1980s, as a prelude to chemoprevention, we rigorously investigated the fascinating target site specific actions of tamoxifen. Human breast tumours implanted in athymic mice did not grow 55 with tamoxifen despite the fact that tamoxifen is an oestrogen in the mouse 5. But oestrogen is needed to maintain bone, so what would the value be of preventing a few breast cancers in a thousand post-menopausal women per year if hundreds of women subsequently developed osteoporosis? To our surprise both tamoxifen and raloxifene (an abandoned breast cancer drug called keoxifene) both maintained ovariectomized rat bone density 75 and prevented carcinogen-induced mammary cancers in a rat model 51. Tamoxifen was better than raloxifene at suppressing mammary tumour appearance 51. This is because tamoxifen has a long biological half-life producing optimal tumour suppression whereas raloxifene is a polyphenolic compound that is rapidly cleared and short acting.
The SERM concept applied to clinical practice was proposed in the last paragraph of the Cain Memorial Lecture in 1990 26. This roadmap for industry is reproduced in the last section, Retrospective and conclusions.
Clinical translation
The animal study of rat bone density with tamoxifen translated to building bone in post-menopausal women 76. Raloxifene was approved to prevent osteoporosis but prevented breast cancer at the same time 77. The chemoprevention trial Study of Tamoxifen and Raloxifene (STAR) showed that both SERMs were able to prevent breast cancer in high risk post-menopausal women by 50% during treatment 78 but after therapy stopped at 5 years tamoxifen maintained chemoprevention of breast cancer but raloxifene did not 79. These clinical results echoed our laboratory study in animals 20 years earlier 51. Raloxifene is recommended to be taken indefinitely to maintain chemoprevention of breast cancer. Perhaps hundreds of thousands of lives have been improved.
The evolution of acquired resistance to tamoxifen
Laboratory model
The serial retransplantation of MCF-7 breast tumours with acquired resistance to tamoxifen into tamoxifen treated mice passes through two phases: Phase I acquired resistance occurs in the ER+ tumour within 1–2 years of tamoxifen treatment. Acquired resistant tumours are characterized as being stimulated to grow with either physiologic oestrogen or tamoxifen 53. No oestrogen or tamoxifen treatment or treatment with a pure anti-oestrogen stops tumour growth 54. Phase II acquired resistance develops with retransplantation after 3–5 years, but now tamoxifen stimulates tumour growth but physiological oestrogen causes tumour regression 61.
Clinical outcome
Low dose oestrogen causes a 30% benefit rate after a woman's tumour becomes resistant to long term adjuvant aromatase inhibitor treatment 80. Most provocatively, the new science of oestrogen-induced apoptosis could be the reason for dramatic decreases in mortality after adjuvant tamoxifen is stopped. Recent data demonstrate that 10 years of tamoxifen is superior to 5 years of tamoxifen 81 but mortality is decreased by 50% compared with historical no treatment data but only in the decade after 10 years of tamoxifen is stopped. Oestrogen-induced apoptosis is also offered as the reason 82 mortality decreases with oestrogen alone treatment as hormone replacement therapy in 60 year old post-menopausal women following a decade of oestrogen deprivation following menopause. It may be that this research strategy leads to new and safer hormone replacement therapy for post-menopausal women.
Retrospective and conclusions
Looking back at this point in our tale, it can be predicted that this will not be the end at all, but the beginning of a new phase of a conversation with Nature. The outcomes of that conversation may determine the next advance in therapeutics.
What started out with a desire to contribute to the development of a medicine to treat cancer seemed, on reflection now, a forlorn hope 40 years ago 27 but I did not realize that at the time (fortunately)! The formula for a successful outcome in my quest to contribute, depended on two principal factors: a willingness to learn new laboratory techniques using relevant animal models that turned out to have significance for translational research in therapeutics and the willingness of innovative and committed individuals in industry and Yorkshire Cancer Research to invest in a young investigator 27. This was followed by the generosity of a philanthropic organization, the Lynn Sage Breast Cancer Foundation in Chicago, who raised a million dollars a year for a decade for my tamoxifen team to define and understand the new science oestrogen-induced apoptosis 61–67.
As a pharmacologist, I defined my goal – use models to discover mechanisms and develop new medicines. Animal models were the key to that success. At the start, the use of long term adjuvant tamoxifen therapy was counterintuitive to the clinical community. Tamoxifen was only effective in controlling the growth of metastatic breast cancer for a year or two 22,23 so it would be dangerous at worst, and unwise at best, to extend adjuvant tamoxifen beyond a year. But micrometastases are clearly different from larger metastatic lesions and a different pharmacology pertains. Perhaps millions of women benefited. There was no clinical understanding of the relevance of the mixed oestrogenic/anti-oestrogenic effects of tamoxifen. In the clinical lectures, I called it the ‘oestrogenic tickle of tamoxifen’. The laboratory finding that tamoxifen selectively blocks oestrogen stimulated breast tumour growth but enhances the growth of pre-existing occult endometrial cancer changed all that 56. Medical practice changed, gynaecologists were involved in breast cancer patient care and major medical problems were avoided that could have killed the patient without appropriate pre-emptive action. A cluster of consistent findings 51,52,55,56,75 by my tamoxifen team at Wisconsin (1980–1993) resulted in the group of medicines, the SERMs.
The idea that a ‘non-steroidal anti-oestrogen’ could switch on or switch off oestrogen target sites around the body could not have been anticipated without animal models to demonstrate antitumour action in the rat mammary gland 43,44,51 but oestrogen-like activity in ovariectomized rat bone 75. This led to a road map for industry 26 as stated in my Bruce F. Cain Award and Memorial Lecture in 1989:
‘Is this the end of the possible applications for anti-oestrogens? Certainly not. We have obtained valuable clinical information about this group of drugs that can be applied in other disease states. Research does not travel in straight lines and observations in one field of science often become major discoveries in another. Important clues have been garnered about the effects of tamoxifen on bone and lipids so it is possible that derivatives could find targeted applications to retard osteoporosis or atherosclerosis. The ubiquitous application of novel compounds to prevent diseases associated with the progressive changes after menopause may, as a side effect, significantly retard the development of breast cancer. The target population would be post-menopausal women in general, thereby avoiding the requirement to select a high risk group to prevent breast cancer’.
The declaration resulted in a whole new drug group that overall, aids women's health. Millions of women continue to benefit.
Lastly, the creation of an animal model of acquired tamoxifen resistance of breast cancer informed us about the unique nature of tamoxifen-stimulated tumour growth. However, the then disheartening fact that this tumour model could not be transferred to cell culture, but demanded constant retransplantation into subsequent generations of tamoxifen treated athymic mice, opened the door to a discovery. Little did we suspect at the beginning, that this routine, labour-intensive procedure, would cause the tumours to evolve through continuing selection pressure over the years. Acquired resistance changed after a couple of years. Tamoxifen treatment caused acquired resistance with either tamoxifen or oestogen-stimulated growth. At 3–5 years of transplantation now the new tamoxifen resistant cell population responded to physiologic oestrogen with tumour regression. It is possible that a woman's own oestrogen does exactly the same to execute prepared micropopulations of tamoxifen resistant cells after 5 years of adjuvant tamoxifen stops 60. Based on the original animal studies demonstrating the evolution of acquired resistance to tamoxifen, subsequent cellular models were used to decipher the molecular events involved in oestrogen-induced apoptosis 65,83–86. This knowledge became pre-positioned in the refereed literature so that the paradoxical finding of fewer breast cancers reported in the oestrogen alone clinical trial of the WHI with a reduction of mortality were understood. Select women lived 82 but the finding that a combination of oestrogen plus a synthetic progestin, which causes an increase in breast cancer incidence, now demands understanding. Resolution of mechanisms and the creation of a safer hormone replacement therapy that prevents breast cancer may indeed be the next chapter of the tamoxifen tale that affects the lives of millions of women worldwide.
However, it would be, perhaps, misleading to imply that human disease can always be modelled successfully with animal equivalents. There is, for example, no animal model for human breast cancer that faithfully replicates outcomes. Focusing on the pharmacology of tamoxifen, but bearing in mind this is just the tip of the iceberg of all medicines, a number of uncertainties and problems persist. To be successful as a therapeutic agent, the medicine must be taken for perhaps a decade or more as a treatment or as a chemopreventive agent in high risk women. Regrettably, and predictably, one of the major side effects of tamoxifen that reduces compliance is menopausal symptoms, particularly hot flushes. Decreases in compliance result in lives lost 87. These are no satisfactory laboratory models to predict this in the clinic. Nevertheless, changes in patient care may be possible. A new combination of the SERM bazedoxifene plus conjugated oestrogen has recently been approved by the Food and Drug Administration in America for the treatment of osteoporosis or menopausal symptoms 88. It seems that the oestrogen can win in the brain to ameliorate menopausal symptoms, but the SERM prevents oestrogen induced breast and endometrial cancer. The combination has an additive effect in bone, an effect first noted with both tamoxifen and raloxifene in animals 75! Metabolism and pharmacodynamics remain a challenge in the two way conversation between laboratory animal results and clinical trials. Although algorithms are available, to model dosage modifications in animals is often not precise. Additionally blood concentrations and metabolites are not consistent between human and other species 89. One long running controversy has been the genotyping of patients for CYP2D6 that governs the available levels of endoxifen in tamoxifen treated patients. The technical issues have recently been reviewed 90 but the simple theory that only higher levels of metabolically produced endoxifen will produce optimal results, can really only be addressed in cell culture. Animal modelling is not possible 89. However, cell culture only provides data on a transient moment in the life of tumour cells and not the shifting adaptive populations that evolve over years of treatment.
As a science, our exploration evolves by trial and error as we meet each new challenge in selective toxicity. In cancer research there has been in the past decade, a huge shift to genetically engineered mice to answer the question ‘is this gene significant?’ At the other extreme is the continuous sequencing of human tumour types to discover patterns and vulnerabilities. However, human tumour data are a single ‘snapshot’ but what human cancer is, is a relentless journey of immense possibilities to overwhelm the human host. This remains hard to model if we subscribe to the mantra that every tumour is different and that only personalized medicine is the way of the future. Tamoxifen with its target of the tumour ER was the first personalized medicine in cancer. Now we have the challenge of navigating out of the Pandora's box we opened.
Professor Paul Ehrlich chose to view the selective targeting of a chemical therapy to cure disease as the search for the ‘Magic Bullet’. Tamoxifen can, in retrospect, be viewed as the discovery of a ‘Magic Machine Gun’, as no other chemical therapy for cancer is used to treat all stages of breast cancer, ductal carcinoma in situ (DCIS), male breast cancer and can be used for the prevention of breast cancer, all by targeting the ER 91, but the ER target around a patient's body can be switched on or switched off selectively by tamoxifen. So, a search for new medicines gave us SERMs. Broad improvements in women's health by selective modulation of the same target in different tissues was an unanticipated consequence of ‘anti-oestrogenic’ treatment. Appropriate animal models significantly advanced health for millions of women to live longer and healthier lives. Mothers see their children grow up, children experience the affection of a grandmother.
Competing Interests
There are no competing interests to declare.
This article is dedicated to my late friend and supporter Sir James Black FRS. The inspiration to create this article occurred when the American Society of Clinical Oncology chose to select my contributions in translational research to be one of the 50 Oncology Luminaries http://cancerprogress.net/node/2086 and, simultaneously, Ms Elodie Picard, a veterinary student from Brussels enquired about my views on the use of animal models in medical research. This is the result. I thank my generations of members of my tamoxifen teams who used laboratory models to transform ideas into lives saved. I thank Fadeke Agboke and Russell McDaniel for their invaluable assistance with this manuscript. This work (VCJ) was supported by the Department of Defense Breast Program under Award number W81XWH-06-1-0590 Center of Excellence; the Susan G Komen For The Cure Foundation under Award number SAC100009; GHUCCTS CTSA (Grant # UL1RR031975) and the Lombardi Comprehensive Cancer Center Support Grant (CCSG) Core Grant NIH P30 CA051008. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Additionally, the views and opinions of the author(s) do not reflect those of the US Army or the Department of Defense.
References
- Geison GL. The Private Science of Louis Pasteur. First edn. Princeton, NJ: Princeton University Press; 1995. [DOI] [PubMed] [Google Scholar]
- Baumler E. Paul Ehrlich: Scientist for Life. First edn. New York, NY: Holmes & Meier Pub; 1984. p. 288. [Google Scholar]
- Black J. Drugs from emasculated hormones: the principle of syntopic antagonism. Science. 1989;245:486–493. doi: 10.1126/science.2569237. [DOI] [PubMed] [Google Scholar]
- Carter SB. Effects of cytochalasins on mammalian cells. Nature. 1967;213:261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Harper MJ, Walpole AL. A new derivative of triphenylethylene: effect on implantation and mode of action in rats. J Reprod Fertil. 1967;13:101–119. doi: 10.1530/jrf.0.0130101. [DOI] [PubMed] [Google Scholar]
- Barrett AM. Cardiac beta-adrenoceptor blockade: the quest for selectivity. J Pharmacol. 1985;16(Suppl 2):95–108. [PubMed] [Google Scholar]
- Emanuel M, Rawlins M, Duff G, Breckenridge A. Thalidomide and its sequelae. Lancet. 2012;380:781–783. doi: 10.1016/S0140-6736(12)60468-1. [DOI] [PubMed] [Google Scholar]
- McBride WG. Thalidomide and congenital abnormalities. Lancet. 1961;ii:1358. [Google Scholar]
- Dally A. Thalidomide: was the tragedy preventable? Lancet. 1998;351:1197–1199. doi: 10.1016/S0140-6736(97)09038-7. [DOI] [PubMed] [Google Scholar]
- Greaves P, Goonetilleke R, Nunn G, Topham J, Orton T. Two-year carcinogenicity study of tamoxifen in Alderley Park Wistar-derived rats. Cancer Res. 1993;53:3919–3924. [PubMed] [Google Scholar]
- Early Breast Cancer Trialists' Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet. 1992;339:71–85. [PubMed] [Google Scholar]
- Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan VC. What if tamoxifen (ICI 46,474) had been found to produce rat liver tumors in 1973? A personal perspective. Ann Oncol. 1995;6:29–34. doi: 10.1093/oxfordjournals.annonc.a059035. [DOI] [PubMed] [Google Scholar]
- Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72:7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan VC. Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. 1. Receptor interactions. J Med Chem. 2003;46:883–908. doi: 10.1021/jm020449y. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. 2. Clinical considerations and new agents. J Med Chem. 2003;46:1081–1111. doi: 10.1021/jm020450x. [DOI] [PubMed] [Google Scholar]
- Phillips DH. Understanding the genotoxicity of tamoxifen? Carcinogenesis. 2001;22:839–849. doi: 10.1093/carcin/22.6.839. [DOI] [PubMed] [Google Scholar]
- Schrek R. The Year Book of Pathology and Clinical Pathology. Chicago: The Year Book Publishers; 1959. Fashions in cancer research; pp. 26–39. [Google Scholar]
- Haddow A, Watkinson JM, Paterson E, Koller PC. Influence of synthetic oestrogens on advanced malignant disease. Br Med J. 1944;2:393–398. doi: 10.1136/bmj.2.4368.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow A, David A. Karnofsky memorial lecture. Thoughts on chemical therapy. Cancer. 1970;26:737–754. doi: 10.1002/1097-0142(197010)26:4<737::aid-cncr2820260402>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI 46474. Br J Cancer. 1971;25:270–275. doi: 10.1038/bjc.1971.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle JN, Ahmann DL, Green SJ, Edmonson JH, Bisel HF, Kvols LK, Nichols WC, Creagan ET, Hahn RG, Rubin J, Frytak S. Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N Engl J Med. 1981;304:16–21. doi: 10.1056/NEJM198101013040104. [DOI] [PubMed] [Google Scholar]
- Walpole AL, Paterson E. Synthetic oestrogens in mammary cancer. Lancet. 1949;2:783–786. doi: 10.1016/s0140-6736(49)91370-7. [DOI] [PubMed] [Google Scholar]
- Jordan VC. The development of tamoxifen for breast cancer therapy: a tribute to the late Arthur L. Walpole. Breast Cancer Res Treat. 1988;11:197–209. doi: 10.1007/BF01807278. [DOI] [PubMed] [Google Scholar]
- Lerner LJ, Jordan VC. Development of antiestrogens and their use in breast cancer: eighth Cain memorial award lecture. Cancer Res. 1990;50:4177–4189. [PubMed] [Google Scholar]
- Jordan VC. Tamoxifen as the first targeted long term adjuvant therapy for breast cancer. Endocr Relat Cancer. 2014;21:R235–246. doi: 10.1530/ERC-14-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan VC. Fourteenth Gaddum Memorial Lecture. A current view of tamoxifen for the treatment and prevention of breast cancer. Br J Pharmacol. 1993;110:507–517. doi: 10.1111/j.1476-5381.1993.tb13840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan VC. Tamoxifen (ICI 46,474) as a targeted therapy to treat and prevent breast cancer. Br J Pharmacol. 2006;147(Suppl. 1):S269–276. doi: 10.1038/sj.bjp.0706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov PY, McDaniel RE, Jordan VC. Basel: Springer; 2013. Tamoxifen: pioneering medicine in breast cancer. [Google Scholar]
- Jordan VC, editor. Estrogen Action, Selective Estrogen Receptor Modulators and Women's Health: Progress and Promise. London: Imperial College Press; 2013. [Google Scholar]
- Jordan VC. A century of deciphering the control mechanisms of sex steroid action in breast and prostate cancer: the origins of targeted therapy and chemoprevention. Cancer Res. 2009;69:1243–1254. doi: 10.1158/0008-5472.CAN-09-0029. [DOI] [PubMed] [Google Scholar]
- Ward HW. Anti-oestrogen therapy for breast cancer: a trial of tamoxifen at two dose levels. Br Med J. 1973;1:13–14. doi: 10.1136/bmj.1.5844.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire WL, Carbone PP, Vollmer EP, editors. Estrogen Receptors in Human Breast Cancer. New York, NY: Raven Press; 1975. [Google Scholar]
- Morgan LR, Jr, Schein PS, Woolley PV, Hoth D, Macdonald J, Lippman M, Posey LE, Beazley RW. Therapeutic use of tamoxifen in advanced breast cancer: correlation with biochemical parameters. Cancer Treat Rep. 1976;60:1437–1443. [PubMed] [Google Scholar]
- Kiang DT, Kennedy BJ. Tamoxifen (antiestrogen) therapy in advanced breast cancer. Ann Intern Med. 1977;87:687–690. doi: 10.7326/0003-4819-87-6-687. [DOI] [PubMed] [Google Scholar]
- Huggins C, Grand LC, Brillantes FP. Mammary cancer induced by a single feeding of polymucular hydrocarbons, and its suppression. Nature. 1961;189:204–207. doi: 10.1038/189204a0. [DOI] [PubMed] [Google Scholar]
- Welsch CW. Host factors affecting the growth of carcinogen-induced rat mammary carcinomas: a review and tribute to Charles Brenton Huggins. Cancer Res. 1985;45:3415–3443. [PubMed] [Google Scholar]
- Jordan VC, Koerner S. Tamoxifen (ICI 46,474) and the human carcinoma 8S oestrogen receptor. Eur J Cancer. 1975;11:205–206. doi: 10.1016/0014-2964(75)90119-x. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Use of the DMBA-induced rat mammary carcinoma system for the evaluation of tamoxifen as a potential adjuvant therapy. Rev Endocr Rel Cancer. 1978:49–55. ; (Oct): [Google Scholar]
- Jordan VC, Dix CJ, Allen KE. The effectiveness of long term tamoxifen treatment in a laboratory model for adjuvant hormone therapy of breast cancer. In: Salmon S, Jones S, editors. Adjuvant Therapy of Cancer II. New York: Grune & Stratton Inc; 1979. pp. 19–26. [Google Scholar]
- Jordan VC, Allen KE. Evaluation of the antitumour activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma model. Eur J Cancer. 1980;16:239–251. doi: 10.1016/0014-2964(80)90156-5. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Antitumour activity of the antioestrogen ICI 46,474 (tamoxifen) in the dimethyl benzanthracene (DMBA)-induced rat mammary carcinoma model. J Steroid Biochem. 1974;5:354. [Google Scholar]
- Jordan VC. Effect of tamoxifen (ICI 46,474) on initiation and growth of DMBA-induced rat mammary carcinomata. Eur J Cancer. 1976;12:419–424. doi: 10.1016/0014-2964(76)90030-x. [DOI] [PubMed] [Google Scholar]
- Giovanella BC, Stehlin JS, Jr, Williams LJ, Jr, Lee SS, Shepard RC. Heterotransplantation of human cancers into nude mice: a model system for human cancer chemotherapy. Cancer. 1978;42:2269–2281. doi: 10.1002/1097-0142(197811)42:5<2269::aid-cncr2820420527>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Soule HD, Vazguez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973;51:1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Brooks SC, Locke ER, Soule HD. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J Biol Chem. 1973;248:6251–6253. [PubMed] [Google Scholar]
- Soule HD, McGrath CM. Estrogen responsive proliferation of clonal human breast carcinoma cells in athymic mice. Cancer Lett. 1980;10:177–189. doi: 10.1016/0304-3835(80)90042-7. [DOI] [PubMed] [Google Scholar]
- Shafie SM, Grantham FH. Role of hormones in the growth and regression of human breast cancer cells (MCF-7) transplanted into athymic nude mice. J Natl Cancer Inst. 1981;67:51–56. [PubMed] [Google Scholar]
- Osborne CK, Hobbs K, Clark GM. Effect of estrogens and antiestrogens on growth of human breast cancer cells in athymic nude mice. Cancer Res. 1985;45:584–590. [PubMed] [Google Scholar]
- Gottardis MM, Jordan VC. Antitumor actions of keoxifene and tamoxifen in the N-nitrosomethylurea-induced rat mammary carcinoma model. Cancer Res. 1987;47:4020–4024. [PubMed] [Google Scholar]
- Gottardis MM, Robinson SP, Jordan VC. Estradiol-stimulated growth of MCF-7 tumors implanted in athymic mice: a model to study the tumoristatic action of tamoxifen. J Steroid Biochem. 1988;30:311–314. doi: 10.1016/0022-4731(88)90113-6. [DOI] [PubMed] [Google Scholar]
- Gottardis MM, Jordan VC. Development of tamoxifen-stimulated growth of MCF-7 tumors in athymic mice after long-term antiestrogen administration. Cancer Res. 1988;48:5183–5187. [PubMed] [Google Scholar]
- Gottardis MM, Jiang SY, Jeng MH, Jordan VC. Inhibition of tamoxifen-stimulated growth of an MCF-7 tumor variant in athymic mice by novel steroidal antiestrogens. Cancer Res. 1989;49:4090–4093. [PubMed] [Google Scholar]
- Jordan VC, Robinson SP. Species-specific pharmacology of antiestrogens: role of metabolism. Fed Proc. 1987;46:1870–1874. [PubMed] [Google Scholar]
- Gottardis MM, Robinson SP, Satyaswaroop PG, Jordan VC. Contrasting actions of tamoxifen on endometrial and breast tumor growth in the athymic mouse. Cancer Res. 1988;48:812–815. [PubMed] [Google Scholar]
- Harper MJ, Walpole AL. Contrasting endocrine activities of cis and trans isomers in a series of substituted triphenylethylenes. Nature. 1966;212:87. doi: 10.1038/212087a0. [DOI] [PubMed] [Google Scholar]
- Terenius L. Structure-activity relationships of anti-oestrogens with regard to interaction with 17-beta-oestradiol in the mouse uterus and vagina. Acta Endocrinol (Copenh) 1971;66:431–447. doi: 10.1530/acta.0.0660431. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Selective estrogen receptor modulation: a personal perspective. Cancer Res. 2001;61:5683–5687. [PubMed] [Google Scholar]
- Wolf DM, Jordan VC. A laboratory model to explain the survival advantage observed in patients taking adjuvant tamoxifen therapy. Recent Results Cancer Res. 1993;127:23–33. doi: 10.1007/978-3-642-84745-5_4. [DOI] [PubMed] [Google Scholar]
- Yao K, Lee ES, Bentrem DJ, England G, Schafer JI, O'Regan RM, Jordan VC. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:2028–2036. [PubMed] [Google Scholar]
- Chisamore MJ, Ahmed Y, Bentrem DJ, Jordan VC, Tonetti DA. Novel antitumor effect of estradiol in athymic mice injected with a T47D breast cancer cell line overexpressing protein kinase C alpha. Clin Cancer Res. 2001;7:3156–3165. [PubMed] [Google Scholar]
- Liu H, Lee ES, Gajdos C, Pearce ST, Chen B, Osipo C, Loweth J, McKian K, De Los Reyes A, Wing L, Jordan VC. Apoptotic action of 17beta-estradiol in raloxifene-resistant MCF-7 cells in vitro and in vivo. J Natl Cancer Inst. 2003;95:1586–1597. doi: 10.1093/jnci/djg080. [DOI] [PubMed] [Google Scholar]
- Osipo C, Gajdos C, Liu H, Chen B, Jordan VC. Paradoxical action of fulvestrant in estradiol-induced regression of tamoxifen-stimulated breast cancer. J Natl Cancer Inst. 2003;95:1597–1608. doi: 10.1093/jnci/djg079. [DOI] [PubMed] [Google Scholar]
- Lewis JS, Meeke K, Osipo C, Ross EA, Kidawi N, Li T, Bell E, Chandel NS, Jordan VC. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97:1746–1759. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]
- O'Regan RM, Gajdos C, Dardes RC, De Los Reyes A, Park W, Rademaker AW, Jordan VC. Effects of raloxifene after tamoxifen on breast and endometrial tumor growth in athymic mice. J Natl Cancer Inst. 2002;94:274–283. doi: 10.1093/jnci/94.4.274. [DOI] [PubMed] [Google Scholar]
- O'Regan RM, Osipo C, Ariazi E, Lee ES, Meeke K, Morris C, Bertucci A, Sarker MA, Grigg R, Jordan VC. Development and therapeutic options for the treatment of raloxifene-stimulated breast cancer in athymic mice. Clin Cancer Res. 2006;12:2255–2263. doi: 10.1158/1078-0432.CCR-05-2584. [DOI] [PubMed] [Google Scholar]
- Jordan VC, Dowse LJ. Tamoxifen as an anti-tumour agent: effect on oestrogen binding. J Endocrinol. 1976;68:297–303. doi: 10.1677/joe.0.0680297. [DOI] [PubMed] [Google Scholar]
- Jordan VC, Jaspan T. Tamoxifen as an anti-tumour agent: oestrogen binding as a predictive test for tumour response. J Endocrinol. 1976;68:453–460. doi: 10.1677/joe.0.0680453. [DOI] [PubMed] [Google Scholar]
- Jordan VC, Collins MM, Rowsby L, Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977;75:305–316. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- Hardell L. Tamoxifen as risk factor for carcinoma of corpus uteri. Lancet. 1988;2:563. doi: 10.1016/s0140-6736(88)92675-x. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Tamoxifen and endometrial cancer. Lancet. 1988;2:1019. doi: 10.1016/s0140-6736(88)90765-9. [DOI] [PubMed] [Google Scholar]
- Fornander T, Rutqvist LE, Cedermark B, Glas U, Mattsson A, Silfversward C, Skoog L, Somell A, Theve T, Wilking N, Askergren J, Hjalmarm l. Adjuvant tamoxifen in early breast cancer: occurrence of new primary cancers. Lancet. 1989;1:117–120. doi: 10.1016/s0140-6736(89)91141-0. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Tamoxifen and endometrial cancer. Lancet. 1989;1:733–734. doi: 10.1016/s0140-6736(89)92255-1. [DOI] [PubMed] [Google Scholar]
- Jordan VC, Phelps E, Lindgren JU. Effects of anti-estrogens on bone in castrated and intact female rats. Breast Cancer Res Treat. 1987;10:31–35. doi: 10.1007/BF01806132. [DOI] [PubMed] [Google Scholar]
- Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, Carbone PP, DeMets DL. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326:852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, Lippman ME, Black D, Glusman JE, Costa A, Jordan VC. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple outcomes of raloxifene evaluation. JAMA. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Jr, Wade JL, 3rd, Robidoux A, Margolese RG, James J, Lippman SM, Runowicz CD, Ganz PA, Reis SE, McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Wade JL, 3rd, Robidoux A, Margolese RG, James J, Runowicz CD, Ganz PA, Reis SE, McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res. 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MJ, Gao F, Dehdashti F, Jeffe DB, Marcom PK, Carey LA, Dickler MN, Silverman P, Fleming GF, Kommareddy A, Jamalabadi-Majidi S, Crowder R, Siegel BA. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302:774–780. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A, Bonfill X, Bradbury J, Clarke M, Collins R, Davis SR, Delmestri A, Forbes JF, Haddad P, Hou MF, Inbar M, Khaled H, Kielanowska J, Kwan WH, Mathew BS, Mittra I, Muller B, Nicolucci A, Peralta O, Pernas F, Petruzelka L, Pienkowski T, Radhika R, Rajan B, Rubach MT, Tort S, Urrutia G, Valentini M, Wang Y, Peto R. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GL, Chlebowski RT, Aragaki AK, Kuller LH, Manson JE, Gass M, Bluhm E, Connelly S, Hubbell FA, Lane D, Martin L, Ockene J, Rohan T, Schenken R, Wactawski-Wende J. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women's Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476–486. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariazi EA, Cunliffe HE, Lewis-Wambi JS, Slifker MJ, Willis AL, Ramos P, Tapia C, Kim HR, Yerrum S, Sharma CG, Nicolas E, Balagurunathan Y, Ross EA, Jordan VC. Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time. Proc Natl Acad Sci U S A. 2011;108:18879–18886. doi: 10.1073/pnas.1115188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan P, Griffith OL, Agboke FA, Anur P, Zou X, McDaniel RE, Creswell K, Kim SH, Katzenellenbogen JA, Gray JW, Jordan VC. c-Src modulates estrogen-induced stress and apoptosis in estrogen-deprived breast cancer cells. Cancer Res. 2013;73:4510–4520. doi: 10.1158/0008-5472.CAN-12-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan P, Agboke FA, McDaniel RE, Sweeney EE, Zou X, Creswell K, Jordan VC. Inhibition of c-Src blocks oestrogen-induced apoptosis and restores oestrogen-stimulated growth in long-term oestrogen-deprived breast cancer cells. Eur J Cancer. 2014;50:457–468. doi: 10.1016/j.ejca.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obiorah I, Sengupta S, Curpan R, Jordan VC. Defining the conformation of the estrogen receptor complex that controls estrogen-induced apoptosis in breast cancer. Mol Pharmacol. 2014;85:789–799. doi: 10.1124/mol.113.089250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCowan C, Wang S, Thompson AM, Makubate B, Petrie DJ. The value of high adherence to tamoxifen in women with breast cancer: a community-based cohort study. Br J Cancer. 2013;109:1172–1180. doi: 10.1038/bjc.2013.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan VC. A(nother) scientific strategy to prevent breast cancer in postmenopausal women by enhancing estrogen-induced apoptosis? Menopause. doi: 10.1097/GME.0000000000000220. 2014 Mar 10. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov PY, McDaniel RE, Fernandes DJ, Korostyshevskiy VR, Bhatta P, Muerdter T, Flockhart D, Jordan VC. Laboratory examination of CYP2D6 relevance in premenopausal breast cancer patients treated with tamoxifen. Br J Pharmacol doi: 10.1111/bph.12864. . In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauch H, Schwab M. Prediction of tamoxifen outcome by genetic variation of CYP2D6 in post-menopausal women with early breast cancer. Br J Clin Pharmacol. 2014;77:695–703. doi: 10.1111/bcp.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clin Cancer Res. 2003;9:1980–1989. [PubMed] [Google Scholar]
- Cummings SR, Ensrud K, Delmas PD, LaCroix AZ, Vukicevic S, Reid DM, Goldstein S, Sriram U, Lee A, Thompson J, Armstrong RA, Thompson DD, Powles T, Zanchetta J, Kendler D, Neven P, Eastell R. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med. 2010;362:686–696. doi: 10.1056/NEJMoa0808692. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2:205–213. doi: 10.1038/nrd1031. [DOI] [PubMed] [Google Scholar]
- Allen KE, Clark ER, Jordan VC. Evidence for the metabolic activation of non-steroidal antioestrogens: a study of structure-activity relationships. Br J Pharmacol. 1980;71:83–91. doi: 10.1111/j.1476-5381.1980.tb10912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black LJ, Jones CD, Falcone JF. Antagonism of estrogen action with a new benzothiophene derived antiestrogen. Life Sci. 1983;32:1031–1036. doi: 10.1016/0024-3205(83)90935-9. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat Rev Cancer. 2007;7:46–53. doi: 10.1038/nrc2048. [DOI] [PubMed] [Google Scholar]


