Figure 1.
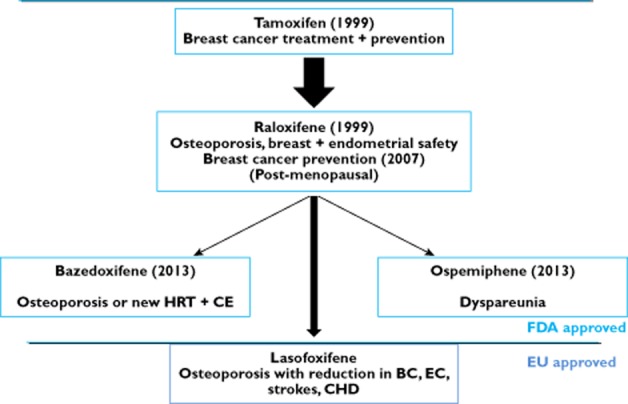
The approvals of individual selective oestrogens receptor modulators (SERMs) in the United States of America through the evaluation system of the Food and Drug Adminisration (FDA). Approvals were specifically for indications at the highest level of toxicologic safety for women without disease but as a new hormone replacement therapy with conjugated oestrogen (HRT + CE) to prevent disease i.e. chemoprevention of osteoporosis, breast cancer (BC), menopausal symptoms or dyspareunia. One SERM, lasofoxifene, was approved for use in the European Union (EU) but was never launched or marketed despite the fact that clinical trials demonstrated a reduction in breast cancer (BC), osteoporosis fracture, strokes, endometrial cancer (EC) and coronary heart disease (CHD) 92
