Figure 4.
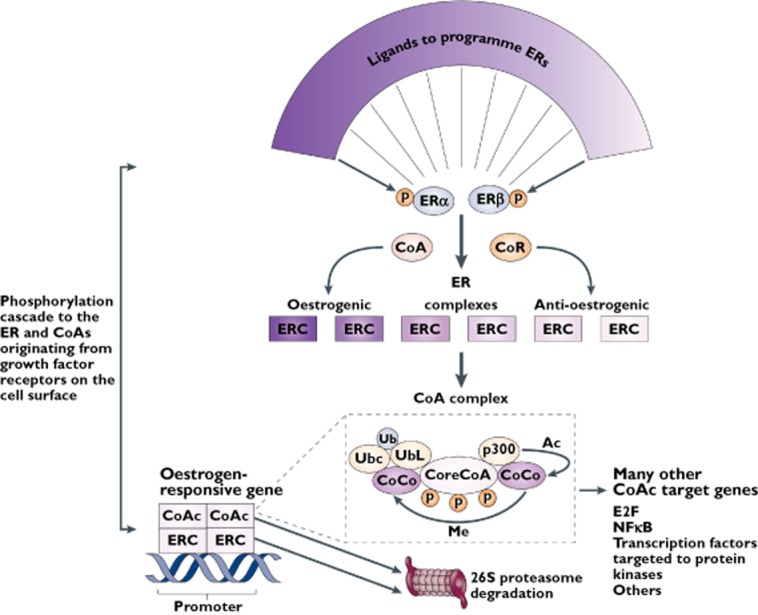
The oestrogen target tissue decision network for selective oestrogen receptor modulation. The shape of the ligands that bind to the oestrogen receptors (ERs) α and β programmes the complex to become an oestrogenic or anti-oestrogenic signal. The context of the ER complex (ERC) can influence the expression of the response through the numbers of co-repressors (CoR) or co-activators (CoA). In simple terms, a site with few CoAs or high levels of CoRs might be a dominant anti-oestrogenic site. However, the expression of oestrogenic action is not simply the binding of the receptor complex to the promoter of the oestrogen-responsive gene, but a dynamic process of CoA complex assembly and destruction. A core CoA, for example, steroid receptor coactivator protein 3 (SRC3), and the ERC are influenced by phosphorylation cascades that phosphorylate target sites on both complexes. The core CoA then assembles an activated multiprotein complex containing specific co-co-activators (CoCo) that might include p300, each of which has a specific enzymatic activity to be activated later. The CoA complex (CoAc) binds to the ERC at the oestrogen-responsive gene promoter to switch on transcription. The CoCo proteins then perform methylation (Me) or acetylation (Ac) to activate dissociation of the complex. Simultaneously, ubiquitiylation by the bound ubiquitin-conjugating enzyme (Ubc) targets ubiquitin ligase (UbL) destruction of protein members of the complex through the 26S proteasome. The ERs are also ubiquitylated and destroyed in the 26S proteasome. Therefore, a regimented cycle of assembly, activation and destruction occurs on the basis of the preprogrammed ER complex. However, the co-activator, specifically SRC3, has ubiquitous action and can further modulate or amplify the ligand-activated trigger through many modulating genes that can consolidate and increase the stimulatory response of the ERC in a tissue. Therefore, the target tissue is programmed to express a spectrum of responses between full oestrogen action and anti-oestrogen action on the basis of the shape of the ligand and the sophistication of the tissue-modulating network. NFkB, nuclear factor kB. Reprinted with permission from the Nature Publishing Group, Jordan 96
