Figure 1.
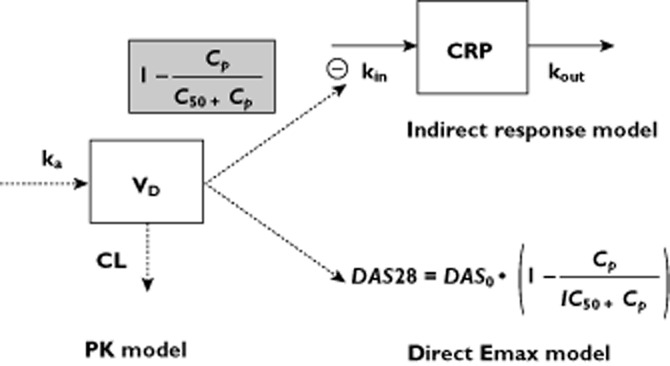
Pharmacokinetic and pharmacokinetic–pharmacodynamic (PK–PD) models. Adalimumab pharmacokinetics was described using a one-compartment model with first-order absorption and elimination rates. The relationship between adalimumab concentrations and C-reactive protein (CRP) levels was described using an indirect model with inhibition of CRP input. The relationship between adalimumab concentrations and the disease activity score in 28 joints (DAS28) was described using a direct Emax inhibitory model. Abbreviations: CL, clearance; Cp, model-predicted adalimumab concentrations; CRP, serum C-reactive protein concentrations; kin, zero-order production rate constant; ka, first-order absorption rate constant; kout, first-order elimination rate constant; C50, adalimumab concentration leading to a 50% decrease of kin; DAS0, DAS28 at baseline, IC50, adalimumab concentration leading to a 50% decrease of DAS0; VD, volume of distribution
