Figure 4.
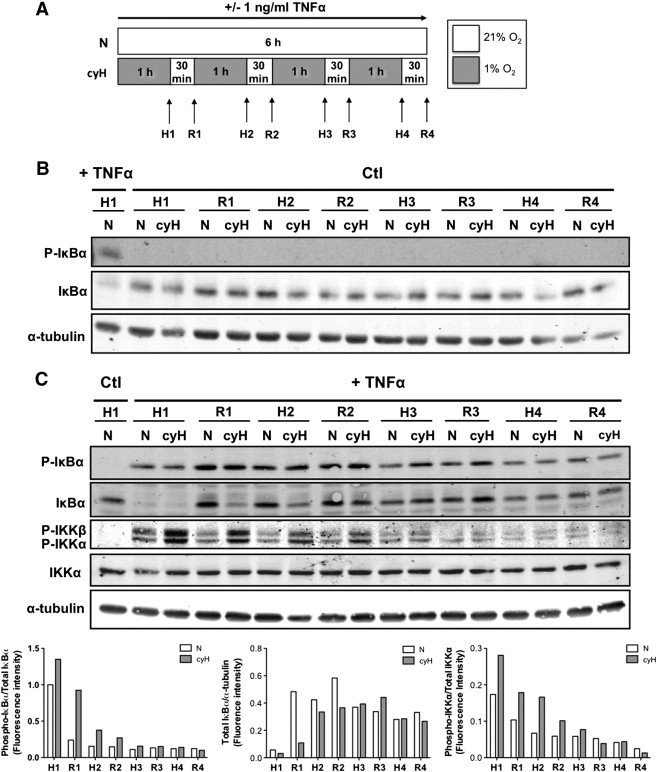
Cycling hypoxia prolongs TNFα-induced NF-κB activation by increasing IKKα/β phosphorylation state, IκBα phosphorylation, and thus its degradation and by slowing down the neosynthesis of IκBα. EAhy926 cells were exposed to N or cyH with or without TNFα (1 ng/ml) for 6 hours. Experimental model is depicted in (A). Phosphorylation of IκBα and IKKα/β and total abundance of IκBα and IKKα were assessed by Western blotting after each step of hypoxia (H1-H2-H3-H4) and reoxygenation (R1-R2-R3-R4) in comparison to N, without (B) or with TNFα (C) (n= 1). α-Tubulin was used as loading control. Bars represent phospho-IκBα fluorescence intensity normalized for total IκBα (left), total IκBα fluorescence intensity normalized for α-tubulin (center) and phospho-IKKα fluorescence intensity normalized for IKKα (right) in the presence of TNFα.
