Fig. 1.
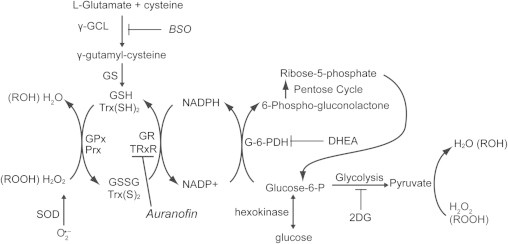
The pathways involving glucose and hydroperoxide metabolism believed to be involved with protection of cancer cells from metabolic oxidative stress (inhibitors of Trx and GSH metabolism are shown in italics). 2DG competes with glucose for uptake into the cells competitively inhibiting pyruvate production and the pentose cycle after glucose-6-phosphate-dehydrogenase (G6PD). DHEA inhibits G6PD. The GSH and Trx dependent systems participate in the detoxification of H2O2 and organic hydroperoxides. NADPH is a source of reducing equivalents for the Trx/GSH-dependent systems. BSO inhibits glutamate cysteine ligase (γ-GCL) preventing glutathione synthesis. Auranofin is the inhibitor of thioredoxin reductase (TrxR), which reduces the oxidized Trx to the reduced form. These inhibitors were used alone and in combination to increase the cancer cell oxidative stress, resulting in cancer cell cytotoxicity.
