Abstract
Scientific evidence supports the use of common reference intervals (RIs) for many general chemistry analytes, in particular those with sound calibration and traceability in place. Already the Nordic countries and United Kingdom have largely achieved harmonised RIs. Following a series of workshops organised by the Australasian Association of Clinical Biochemists (AACB) between 2012 and 2014 at which an evidence-based approach for determination of common intervals was developed, pathology organisations in Australia and New Zealand have reached a scientific consensus on what adult and paediatric intervals we should use across Australasia. The aim of this report is to describe the processes that the AACB and the Royal College of Pathologists of Australasia have taken towards recommending the implementation of a first panel of common RIs for use in Australasia.
Background
One of the current strategic priorities for the Australasian Association of Clinical Biochemists (AACB) is to encourage and assist laboratories to achieve harmonisation of RIs for the common chemistry analytes where sound calibration and traceability are in place. The pathology profession in Australia has been talking about common RIs for some years whereas the Nordic countries and United Kingdom have largely achieved this goal.1,2
During 2004 and 2005 the AACB Committee for Common Reference Intervals worked to promote use of common RIs. Pathologists Graham Jones and Tony Barker toured Australia and New Zealand promoting the widespread adoption of common RIs especially in Australian laboratories whereas in New Zealand harmonised RIs were already in use in the Auckland regional laboratories. However, inertia for harmonisation remained for several more years in Australia.
This was despite studies having shown that the variation in RIs for chemistry analytes may be much greater than the analytical inaccuracy of their measurements. For example, the International Measurement Evaluation Program-17 data for Australian and New Zealand participants showed that both the upper and lower reference limits gave much higher between-laboratory variation than was seen for the measurement results. The data demonstrated that the RIs did not compensate for method differences and had variability unrelated to the measurement.3
In 2010 the AACB Committee for Common Reference Intervals was reformed following a survey of laboratory RIs in which it was blatantly evident that different RIs were in use even for the same methods and manufacturer’s chemistries. Examples of survey results are shown for the lower reference limit for sodium (132–137 mmol/L) and upper reference limit for potassium (4.8–5.5 mmol/L) in Figures 1A and 1B respectively.4 Further evidence for non-harmonised RIs despite similar test results comes from the Royal College of Pathologists of Australasia Quality Assurance Program (RCPAQAP) Liquid Serum Chemistry in 2013 which analysed general chemistry analytes in two normal adult sera and at the same time surveyed laboratory RIs (Figure 1C).5 Hence, laboratories that use the same platforms and same reagents but use different RIs and decision limits can give quite different result interpretations for the same values. Clinical care providers may not be aware of these differences especially if the result transfer from the laboratory to the general practice does not explain any different interpretive criteria applied in setting the RIs. This situation has the potential to worsen a patient’s outcome by causing different clinical interpretation with a risk to the patient of inappropriate over- or under-investigation or treatment.
Figure 1.
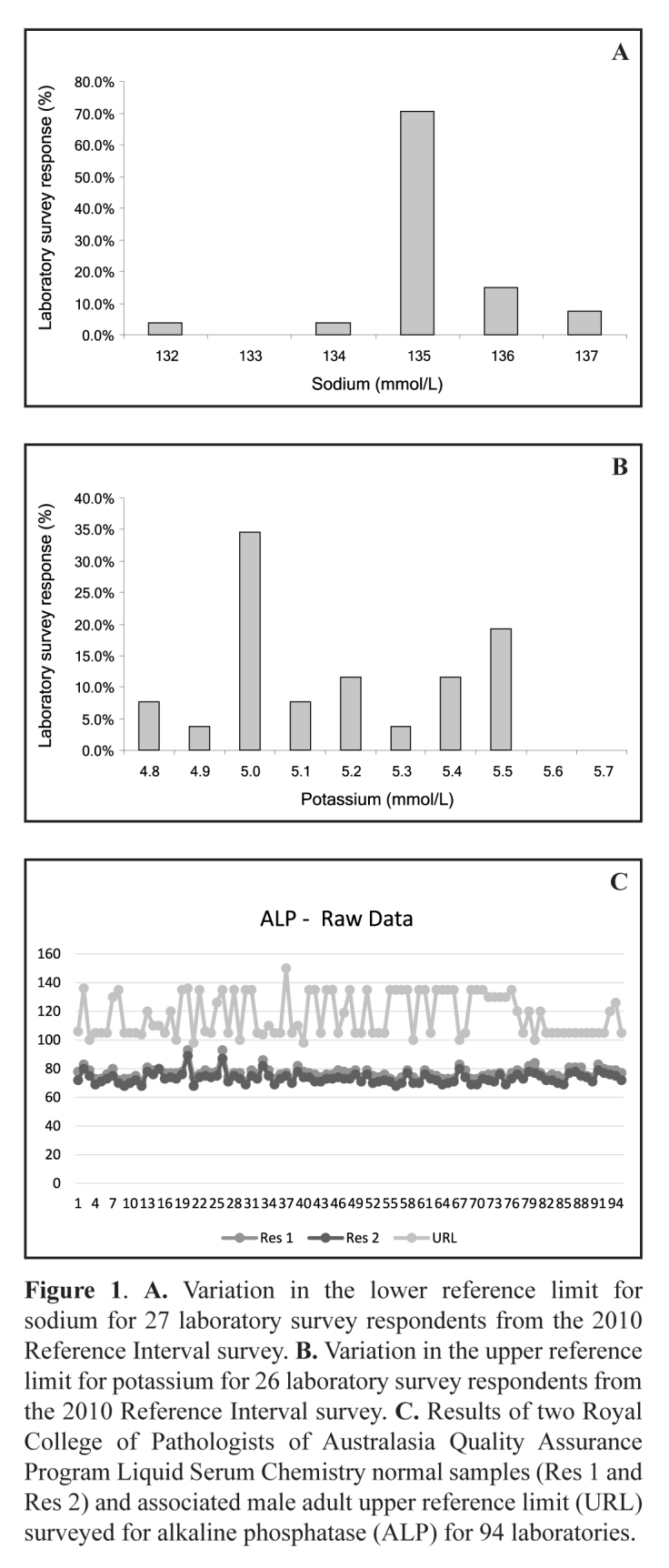
A. Variation in the lower reference limit for sodium for 27 laboratory survey respondents from the 2010 Reference Interval survey. B. Variation in the upper reference limit for potassium for 26 laboratory survey respondents from the 2010 Reference Interval survey. C. Results of two Royal College of Pathologists of Australasia Quality Assurance Program Liquid Serum Chemistry normal samples (Res 1 and Res 2) and associated male adult upper reference limit (URL) surveyed for alkaline phosphatase (ALP) for 94 laboratories.
A key driver for RI harmonisation in Australia has been the electronic health medical record (eHMR) and the desire for the amalgamation of an individual patient’s results from different pathology providers independent of method, unit or RI used. In order to harmonise the reporting of patient pathology results and reduce the confusion in this area for both doctors and patients, an Australian Government-funded project, Pathology Units and Terminology Standardisation (PUTS), was begun by the RCPA in 2011.6,7 The aim was to standardise test units and terminology in pathology.
The aim of this report is to describe the processes that the AACB and the RCPA have taken towards recommending the implementation of a first panel of common RIs for use in Australasia. At the recent 2014 workshop held on April 30 and May 1 in Sydney, it was unanimously agreed by the pathologists and scientists attending the meeting that the following tables of common RIs be implemented (Tables 1 and 2). The common RIs have been formally endorsed by the AACB and the RCPA Board.
Table 1.
Australasian Harmonised Reference Intervals for Adults (AHRIA).*
| Analyte | Male | Female |
|---|---|---|
| Sodium | 135–145 mmol/L | |
| Potassium† | 3.5–5.2 mmol/L | |
| Chloride | 95–110 mmol/L | |
| Bicarbonate | 22–32 mmol/L | |
| Creatinine‡ | 60–110 μmol/L | 45–90 μmol/L |
| Calcium | 2.10–2.60 mmol/L | |
| Calcium (albumin adjusted) | 2.10–2.60 mmol/L | |
| Phosphate§ | 0.75–1.50 mmol/L | |
| Magnesium | 0.70–1.10 mmol/L | |
| Lactate Dehydrogenase [L to P] (IFCC)‖ | 120–250 U/L | |
| Alkaline Phosphatase¶ | 30–110 U/L | |
| Total Protein | 60–80 g/L | |
Unless otherwise specified, the intervals are for serum or plasma for adults (≥18 y). The intervals are for use by laboratories using methods which are traceable to JCTLM-listed reference materials, methods and services (except bicarbonate where no references are listed). Cholesterol, triglycerides, and high-density lipoprotein-cholesterol have decision limits determined by the Australian and New Zealand guidelines.24 Whether the decision limit for fasting glucose should go down to 6.1 mmol/L for the definition of Impaired Fasting Glycaemia (FPG), or down to 5.5 mmol/L for the risk of diabetes (follow up with HbA1c or OGTT), will need communication with diabetes associations in Australia and New Zealand. The bias study indicates that these analytes are largely harmonised.25
This range is proposed for use for both serum and plasma. Laboratories testing only heparin plasma may choose to use a lower interval.
Creatinine has harmonised reference intervals for adults up to the age of 60 y. For older ages laboratories may elect to maintain these.
Starting at age 20 y to align with paediatric intervals.
[L to P] (IFCC), lactate to pyruvate method (IFCC method).
Starting at age 22 y to align with paediatric intervals.
Table 2.
Australasian Harmonised Reference Intervals for Paediatrics (AHRIP).
| Analyte | Age | Reference Interval | ||
|---|---|---|---|---|
| Sodium | 0w to <1w | 132–147 mmol/L | ||
| 1w to <18y | 133–144 mmol/L | |||
| Potassium (serum)* | 0w to <1w | 3.8–6.5 mmol/L | ||
| 1w to <26w | 4.2–6.7 mmol/L | |||
| 26w to <2y | 3.9–5.6 mmol/L | |||
| 2y to <18y | 3.6–5.3 mmol/L | |||
| Potassium (plasma)† | 0w to <1w | 3.5–6.2 mmol/L | ||
| 1w to <26w | 3.8–6.4 mmol/L | |||
| 26w to <2y | 3.5–5.4 mmol/L | |||
| 2y to <18y | 3.3–4.9 mmol/L | |||
| Chloride | 0w to <1w | 98–115 mmol/L | ||
| 1w to <18y | 97–110 mmol/L | |||
| Bicarbonate | 0w to <1w | 15–28 mmol/L | ||
| 1w to <2y | 16–29 mmol/L | |||
| 2y to <10y | 17–30 mmol/L | |||
| 10y to <18y | 20–32 mmol/L | |||
| Creatinine‡ | 0w to <1w | 22–93 μmol/L | ||
| 1w to <4w | 17–50 μmol/L | |||
| 4w to <2y | 11–36 μmol/L | |||
| 2y to <6y | 20–44 μmol/L | |||
| 6y to <12y | 27–58 μmol/L | |||
| Male | Female | |||
| 12y to <15y | 35–83 μmol/L | 12y to <15y | 35–74 μmol/L | |
| 15y to <19y | 50–100 μmol/L | 15y to <19y | 38–82 μmol/L | |
| Calcium | 0w to <1w | 1.85–2.80 mmol/L | ||
| 1w to <26w | 2.20–2.80 mmol/L | |||
| 26w to <2y | 2.20–2.70 mmol/L | |||
| 2y to <18y | 2.20–2.65 mmol/L | |||
| Phosphate | 0w to <1w | 1.25–2.85 mmol/L | ||
| 1w to <4w | 1.50–2.75 mmol/L | |||
| 4w to <26w | 1.45–2.50 mmol/L | |||
| 26w to <1y | 1.30–2.30 mmol/L | |||
| 1y to <4y | 1.10–2.20 mmol/L | |||
| 4y to <15y | 0.90–2.00 mmol/L | |||
| 15y to <18y | 0.80–1.85 mmol/L | |||
| 18y to <20y | 0.75–1.65 mmol/L | |||
| Magnesium | 0w to <1w | 0.60–1.00 mmol/L | ||
| 1w to <18y | 0.65–1.10 mmol/L | |||
| Alkaline Phosphatase | 0w to <1w | 80–380 U/L | ||
| 1w to <4w | 120–550 U/L | |||
| 4w to <26w | 120–650 U/L | |||
| 26w to <2y | 120–450 U/L | |||
| 2y to <6y | 120–370 U/L | |||
| 6y to <10y | 120–440 U/L | |||
| Male | Female | |||
| 10y to <14y | 130–530 U/L | 10y to <13y | 100–460 U/L | |
| 14y to <15y | 105–480 U/L | 13y to <14y | 70–330 U/L | |
| 15y to <17y | 80–380 U/L | 14y to <15y | 50–280 U/L | |
| 17y to <19y | 50–220 U/L | 15y to <16y | 45–170 U/L | |
| 19y to <22y | 45–150 U/L | 16y to <22y | 35–140 U/L | |
The paediatric serum potassium reference intervals are recommended in laboratories (a) with serum primary tubes, or (b) where serum and plasma are used variably in paediatrics as primary tubes.
The paediatric plasma potassium reference intervals are recommended in laboratories (a) with plasma primary tubes, or (b) where serum and plasma are used variably but plasma is the tube of choice in paediatrics.
Reference intervals for patients <19 y are specific for laboratories which use the Ortho Vitros enzymatic creatinine assay. For laboratories that do not use the Vitros enzymatic creatinine assay, the adult creatinine reference interval may be applied from age 18 y.
Evidence-Based Approach to Determination of Common Reference Intervals
1. Processes Adopted
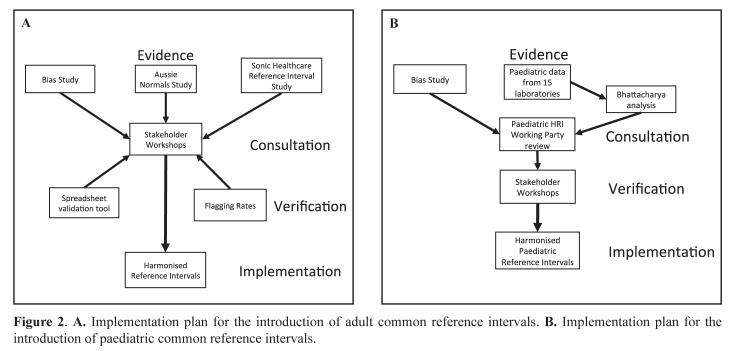
While laboratories are well-trained in method verification and validation to determine if assays are fit-for-purpose, they are less aware of the importance of selecting the most appropriate and evidence-based Reference Intervals for optimal interpretation of results. To better understand the evidence supporting use of common RIs and various physiological factors which affect them, the AACB organised workshops in 2012, 2013 and again in 2014, for representatives from all the major hospitals/networks and pathology organisations in Australia and New Zealand to reach a scientific consensus on what intervals we should use across Australasia. As well, during 2012, pathologist Ken Sikaris toured Australia and New Zealand on the AACB Current Concepts tour to discuss the physiology of RIs.8 The tasks involved in the implementation of harmonisation of RIs across Australasia are documented in Appendix 1. They underscore the importance of a structured organisational plan when tackling the sequence of practical activities that were required to achieve a major change in pathology RIs. Figures 2A and 2B summarise the overall plan for implementation of adult and paediatric common RIs.
Figure 2.
A. Implementation plan for the introduction of adult common reference intervals. B. Implementation plan for the introduction of paediatric common reference intervals.
2. Checklist Approach
At each harmonisation workshop the process of consensus involved talks and breakout discussion sessions being held over 2-day periods and centred on developing the evidence for acceptance of a common or harmonised RI. A checklist assessment process (Table 3) was adopted to assess the evidence for the use of common RIs and was based on the following criteria:9
Define analyte (measurand)
Define assays used, accuracy base, analytical specificity, method-based bias
Consider important pre-analytical differences, actions in response to interference
Define the principle behind the RI (e.g. central 95%)
- Describe evidence for selection of common RIs
- data sources (literature, lab surveys, manufacturer)
- data mining
- bias goal as quality criterion for acceptance
Consider partitioning based on age, sex, etc.
Define degree of rounding
Clinical considerations of the RI
Consider use of common RI
Document and implement.
Table 3.
Checklist approach for development of the evidence for harmonised adult reference intervals (RIs) for 11 chemistry analytes.
| Analyte | Population RI | Units |
JCTLM-listed traceability or preferred method and reference material |
Pre-analytics 1. Serum/plasma 2. Sample collection 3. Interferences |
Analytical differences |
Partitioning by 1. Gender 2. Age |
Reporting Interval |
|---|---|---|---|---|---|---|---|
| Sodium | Based on healthy subjects not hospital patients. | mmol/L | Flame atomic emission spectroscopy. SRM 919 (pure NaCl). SRM 909, 956 (human serum). |
1. Interchangeable. | Analytically there are no meaningful differences (both direct and indirect ISE methods). | None required. | 1 mmol/L |
| Potassium | Based on healthy subjects not hospital patients. | mmol/L | Flame atomic emission spectroscopy. SRM 918 (pure KCl). SRM 909, 956 (human serum). |
1. Serum potassium is 0.3–0.4 mmol/L higher than plasma potassium. A lower cutoff may be more appropriate to plasma samples. Serum is the preferred sample for RI. 2. Delayed sample separation may require that a higher URL is used (e.g. 5.5 mmol/L). 3. Haemolysis. |
Analytically there are no differences (both direct and indirect ISE methods). | None required. | 0.1 mmol/L |
| Chloride | Based on healthy subjects not hospital patients. | mmol/L | Coulometric titration. SRM 918 (pure KCl). SRM 919 (pure NaCl). SRM 909, 956 (human serum). |
1. Interchangeable. | Analytically there are no differences (both direct and indirect ISE methods). | None required. | 1 mmol/L |
| Bicarbonate | Based on healthy subjects not hospital patients. | mmol/L | Not JCTLM-listed. Traceable to Corning blood gas analyser and thermal conductivity methods. Na2CO3 aqueous standard prepared gravimetrically. |
1. Interchangeable (Total CO2 by blood gas analysis is 1 mmol/L different from serum bicarbonate) 2. Venous samples to be specified. |
Architect reads lower than other assays. | None required (increases slightly with age). | 1 mmol/L |
| Creatinine | Based on healthy subjects not hospital patients. eGFR used for decision making. |
μmol/L | ID-GC/MS and ID-LC/MS (some methods require instrument factors). SRM 914 (pure creatinine). SRM 909, 967 (human serum). |
1. Interchangeable. 2. Increases with meat consumption. |
Analytically there are no differences. | 1. Gender differences. 2. Age-related increases above 60 y not agreed by Renal Physicians. |
1 μmol/L10 |
| Calcium/Adjusted Calcium* | Based on healthy subjects not hospital patients. | mmol/L | Flame atomic absorption spectroscopy and ICP-MS. SRM 915a (pure CaCl2). SRM 915b (pure CaCO3). SRM 909, 956 (human serum). |
1. Interchangeable. 2. Increases with posture, tourniquet, haemoconcentration. Fasting has a small effect and a lower URL (2.55 mmol/L) may be appropriate. |
Analytically there are no differences. | None required (Age and gender differences are not clinically relevant). | 0.01 mmol/L |
| Phosphate | Based on healthy subjects not hospital patients. If using paediatric data, adopt adult RI at 20 y. | mmol/L | HPIC. Traceable to ammonium molybdate and phosphomolybdate/p-semidine methods. SRM 200 (pure KH2PO4). SRM 3139 (phosphorous). |
1. Interchangeable. | Vitros is outside minimal bias limits and does not show equivalence for a shared RI (Fig. 3C & 3D). | None required. | 0.01 mmol/L† |
| Magnesium | Based on healthy subjects not hospital patients. | mmol/L | Flame atomic absorption spectroscopy. SRM 929 (pure Mg gluconate). SRM 909, 956 (human serum). |
1. Interchangeable. | Analytically there are no differences. | None required. | 0.01 mmol/L |
| Lactate Dehydrogenase [L to P] IFCC | Based on healthy subjects not hospital patients. Used for monitoring in haematological malignancy. | U/L | IFCC lactate to pyruvate [L to P] reference measurement procedure at 37 °C. | 1. Interchangeable. 3. Haemolysis. |
Methodological differences seen in bias study. Vitros uses pyruvate to lactate [P to L] method. | None required. | 10 U/L‡ |
| Alkaline Phosphatase | Based on healthy subjects not hospital patients. If using paediatric data, adopt Adult RI at 22 y. | U/L | IFCC reference measurement procedure at 37 °C. | 1. Interchangeable. | For most methods there are no differences although Vitros and Olympus are possibly higher. | Some age and gender differences but not clinically significant. | 10 U/L‡ |
| Total Protein | Based on healthy subjects not hospital patients. Globulin estimation considered more important for detecting paraproteinaemias. | g/L | Biuret candidate reference method.11 SRM 927 (7% solution of bovine serum albumin). |
1. Interchangeable. | Analytically there are no differences. | None required. | 1 g/L |
eGFR, estimated glomerular filtration rate; HPIC, high-pressure ion chromatography; ICP-MS, inductively coupled plasma-mass spectrometry; ID-LC/MS, isotope dilution-liquid chromatography/mass spectrometry; ID-GC/MS; isotope dilution-gas chromatography/mass spectrometry; IFCC, International Federation of Clinical Chemistry and Laboratory Medicine; JCTLM, Joint Committee for Traceability in Laboratory Medicine; [L to P], lactate to pyruvate; SRM, standard reference material; URL, upper reference limit.
Adjusted calcium requires that a different formula is used depending whether albumin is measured by bromocresol purple or by bromocresol green.
2 decimal places accepted for phosphate RI at 2014 Harmonisation Workshop until agree to change.
Decile reporting interval of 10 U/L for lactate dehydrogenase and alkaline phosphatase (deciles accepted at 2014 Harmonisation Workshop).
The RI refers to the interval between two reference limits that includes, usually, the central 95% of the reference values for a healthy population of reference individuals. The 95% interval is usually two-sided defined by low and high cut-off values excluding 2.5% of the reference population on each side. For the first panel of 11 chemistry analytes, health-associated population reference limits were determined.
3. Assessment of Method Differences
Method differences were assessed by various means including a bias study using commutable patient-based samples, the RCPAQAP Liquid Serum Chemistry survey, manufacturer’s traceability claims and by the average of normals from local laboratories.
Bias Study
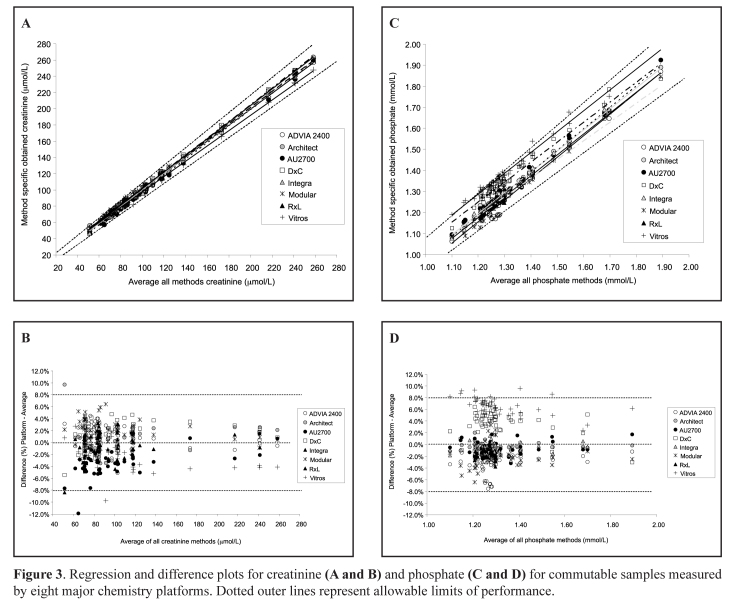
A key requirement for the use of common RIs is a sufficiently small between-method bias. Therefore, a major analytical factor to be considered was the effect of methodological differences on bias and if this would affect the sharing of a common RI. Samples selected from the Aussie Normals study were used to assess the between-method bias of methods (largely within the RI range) for the eight main chemistry platforms (Abbott Diagnostics Architect, Roche Diagnostics Modular and Integra, Beckman Coulter DxC and Olympus, Siemens Healthcare Dimension and Advia, Ortho Clinical Diagnostics Vitros). In August 2011 frozen aliquots of 33 commutable samples from RI subjects were dispatched to 24 laboratories (at least 3 laboratories participated per platform) for analysis of 27 common analytes. These analytes included routine liver and kidney function tests, lipids, iron studies, C-reactive protein, calcium including albumin correction, magnesium, phosphate and urate.12 Also see the separate paper in this publication. The approach taken when analysing the data was to compare the average result for each analyte with the mean of all results. The RCPAQAP allowable limits of performance were used to determine whether bias would prevent the use of a common RI by assessing if all results fell within the allowable limits of agreement and if regression lines were all within the allowable limits for the eight routine measurement procedures that were evaluated.13 The RCPAQAP allowable limits have been derived using biological variability and therefore are well-suited to validation of RIs that are effectively the sum of intra- and inter-individual biological variabilities.13 In Figures 3A and 3B the regression and difference plots for creatinine, a well-standardised analyte, indicate the harmonisation of values measured by the eight platforms. Greater variation was observed for phosphate with Vitros and possibly DxC methods having a positive bias (Figures 3C and 3D).
Figure 3.
Regression and difference plots for creatinine (A and B) and phosphate (C and D) for commutable samples measured by eight major chemistry platforms. Dotted outer lines represent allowable limits of performance.
Calibration Traceability
Manufacturers’ calibration traceability claims were also assessed for method differences.14 An essential requirement for method harmonisation is traceability to available reference materials (pure and/or matrixed) and/or reference measurement procedures. Apart from bicarbonate, all other analytes listed in Table 3 have a complete reference measurement system or a reference measurement procedure listed on the Joint Committee for Traceability in Laboratory Medicine (JCTLM) website.15 Interestingly, the Abbott bicarbonate assay has read low but has now has been restandardised to match results by other manufacturers’ assays.
4. Selection of Reference Intervals
Various sources of information on RIs were searched including local formal RI studies, published studies from the literature, laboratory surveys, manufacturer’s product information, relevant guidelines, and mining of databases. RI selection also considered pre-analytical and partitioning issues and significant figures.
Common Laboratory Usage
In 2013 the RCPAQAP Liquid Serum General Chemistry program was piloted. Participating laboratories were asked to analyse two normal samples collected from blood donors for the general chemistry analytes and to provide their laboratory lower and upper reference limits for these analytes. The survey was designed to assess bias using native patient material and to allow laboratories to compare their RIs with those from other laboratories using the same and different methods. It will serve as a baseline survey to establish the RIs currently being used and will be repeated in subsequent years to assess the uptake of common RIs. See the separate paper in this publication.
Published Values
The Nordic Reference Interval Project (NORIP) established common RIs in apparently healthy adult populations from five Nordic countries for 25 of the most common clinical chemistry analytes (Table 4).1 Importantly, results were traceable to higher-order reference measurement systems. In the UK, reference limits have been established by a survey of RIs in use and form part of the information for harmonising RIs (Table 4).2
Table 4.
Scandinavian, United Kingdom, and Australian and New Zealand adult reference intervals for 11 chemistry analytes.
| Analyte | Unit | NORIP | UK Pathology Harmony | Aussie Normals* | AHRIA |
|---|---|---|---|---|---|
| Sodium | mmol/L | 137–145 | 133–146 | 136–145 | 135–145 |
| Potassium | mmol/L | 3.6–4.6 (serum) | 3.5–5.3 | 3.7–4.9 (plasma) | 3.5–5.2 (serum) |
| Chloride | mmol/L | - | 95–108 | 101–110 | 95–110 |
| Bicarbonate | mmol/L | 22–32 | 22–29 | 20–29‡ | 22–32 |
| Creatinine | μmol/L | M: 60–100 F: 50–90 |
- | M: 55–106 (18–<96y) F: 42–87 (18–<96y) |
M: 60–110 (18–<60y) F: 45–90 (18–<60y) |
| Calcium | mmol/L | 2.17–2.47 (18–<50y) | 2.2–2.6 | 2.19–2.56 | 2.10–2.60 |
| Phosphate | mmol/L | M: 0.75–1.65 (18–<50y) F: 0.85–1.50 (18–<50y) |
0.8–1.5 | 0.85–1.40 | 0.75–1.50 |
| Magnesium | mmol/L | 0.71–0.94 | 0.7–1.0 | 0.77–1.04 | 0.70–1.10 |
| Lactate Dehydrogenase | U/L | 105–205 (18–<70y) | - | 124–231 | 120–250 |
| [L to P] IFCC† | 115–255 (≥70y) | ||||
| Alkaline Phosphatase | U/L | 35–105 | 30–130 | 40–111 | 30–110 |
| Total Protein | g/L | 62–78 (serum) | 60–80 | 62–79 | 60–80 |
NORIP, Nordic Reference Interval Project; AHRIA, Australasian Harmonised Reference Intervals for Adults.
Data supplied by G Koerbin and P Hickman, personal communication.
[L to P] (IFCC), lactate to pyruvate method (IFCC method).
Bicarbonate measured prior to Abbott recalibration
Local Reference Interval Studies
The Australian Aussie Normals study is a direct RI study of 1876 male and female healthy adult Australians from the Australian Capital Territory (ACT) in the age group 18–95 y. All volunteers resided in the Canberra region and completed a questionnaire that included questions associated with lifestyle such as known diseases and medications. Exclusion was based on conditions such as pregnancy, diabetes, renal or cardiovascular disease. Up to 91 biochemistry analytes were measured by Abbott Architect ci8200 and ci16200 analysers. The ACT has a multicultural population which is representative of other populations across Australia. Values from this formal RI study confirm the common RIs being recommended for use in Australia and New Zealand (Table 4; personal communication, G. Koerbin and P. Hickman).
Expert Groups and Data Mining
Several expert groups have provided huge input into the acquisition of age- and gender-partitioned data from birth to old age including: Sonic Healthcare, Auckland Regional Quality Assurance Group (ARQAG), New Zealand South Island QAG (SIQAG), The Alfred Hospital, and PathWest. In particular, pathologist Ken Sikaris and the Sonic Healthcare Chemical Pathology Standard setting group have contributed to the increased understanding of RI partitioning through their data mining of millions of data points from primary care patients. As well, the AACB Paediatric Biochemistry working party, led by pathologist Tina Yen, has analysed over 200,000 paediatric data points provided by 15 Australasian laboratories and developed age- and gender-partitioned RIs for the main general chemistry analytes from birth to 18 years of age. Through analysis of this huge database the working party has determined that there are few platform-related differences in these paediatric RIs. The tools used to analyse the data include assessment of patient medians, flagging rates for proposed intervals and Bhattacharya analysis to determine underlying distributions in the presence of outlier results as well as data cleansing by use of results from patients with a single measurement for an analyte.
Ethnicity
The Aussie Normals data combined with the millions of data points obtained from data mining have incorporated the diversity of multicultural groups living within Australia and New Zealand. If there are ethnic differences e.g. between Caucasians, Asians, Aboriginals or Maoris, which there is little consensus or data available for, laboratories within Australia do not record a patient’s ethnicity when requesting blood tests and would not be seeking this partitioning information. The six cities study in Asia showed that differences are more likely to be due to extrinsic reasons such as diet, climate and socioeconomic factors than intrinsic differences.16
Significant Figures
At the 2014 Harmonisation Workshop it was agreed by consensus that we use harmonised reporting intervals based on deciles to convey the accuracy of results as well as the accuracy of RIs. Reference limit values should be rounded to the same number of decimal places as the measurement uncertainty for an analyte. Values should be expressed to three significant figures within and across decades of values.17,18 Hence, it was agreed at the workshop that in accepting the phosphate RI of 0.75–1.50 mmol/L the profession is accepting two decimal places until there is a consensus to change this.
Clinical Considerations of the RI and Flagging Rates
The proposed reference limits for adults (Table 1) are also supported by flagging rates which provide an indication of the clinical considerations of a RI. Based on the same arbitrary principle of minimum, desirable and optimal categories used to define allowable bias limits, flag rates may range from 1.0% to 1.8% for low flagging rates, and 5.7% to 3.3% for high flagging rates, respectively. However, flag rates may be more complex to interpret depending on the population used to derive them. The typical flagging rates for assays without biases of 2–3% from primary care patients were generally lower and more consistent than the hospital data, mainly because it was a general practice population with private hospital data removed (Table 5). Also, patients with multiple results for an analyte only had the first value kept (repeats are more likely to be abnormal as the reasons for repeating tests in outpatients are usually related to a worsening chronic disease).
Table 5.
Typical flagging rates for the first measurement in outpatient adults (18–60 y) for 11 chemistry analytes.
| Analyte | Flag rate at LRL (%) | Flag rate at URL (%) | Comments |
|---|---|---|---|
| Sodium | 2.5 | 1 | Hypernatraemia is generally uncommon. |
| Potassium | 1 | Generally 2.5 | Higher flag rates may reflect pre-analytical issues e.g. sample transport or haemolysis. |
| Chloride | 1 | <1 | |
| Bicarbonate | 2.5 | <2.5 | Low values by Abbott assay. Manufacturer is recalibrating assay. |
| Creatinine | 2.5 | Generally 2.5 | Higher rates may reflect the problem in removing disease from hospital databases. |
| Calcium | 2.5 | Variable; low in men and premenopausal women; 3.2 % in postmenopausal women. | Reference interval of 2.10–2.60 mmol/L accommodates lot-to-lot calibrator variability. There is no loss of sensitivity of detection of primary hyperthyroidism in postmenopausal women when using 2.60 mmol/L as the URL. |
| Phosphate | 1.5 | 2.5 | Hypophosphataemia was uncommon and probably appropriate to its clinical importance. |
| Magnesium | 5 | 1 | Hypomagnesaemia was more common than expected. The Aussie Normals study had a different cut-off of 0.77 mmol/L. The reference interval is reported to broaden with age.19 |
| Lactate Dehydrogenase | Low | Commonly >2.5 | Vitros and DxC assays have higher low flag rates but this may not be clinically significant. |
| Alkaline Phosphatase | <1 | 7–10 | The benefit of using the URL of 110 U/L is to detect pathology in postmenopausal women. Increasing the URL to 115 U/L had negligible impact because of the logarithmic distribution of values. |
| Total Protein | <2.5 | <3.5 | Rounding of LRL from 62 to 60 g/L was for convenience. Flagging of calculated globulins may detect immune deficiency better. |
LRL, lower reference limit; URL, upper reference limit.
Flag rates for low bicarbonates were very common on the Abbott, averaging 14% compared to Roche at about 4%. Abbott has recalibrated their bicarbonate assay, and the low bias for Abbott should be resolved by the time this article is printed. Hypocalcaemia flags similarly varied between laboratories, probably mainly due to illness and the effect of low albumin on the raw calcium. Hypomagnesaemia was more common than expected (5%), but the only evidence for a different RI was a cut-off of 0.77 mmol/L from the Aussie Normals study which is higher than the recommended 0.70 mmol/L. Low lactate dehydrogenase (LDH) flagging rates were appropriately uncommon, except for Ortho Vitros and Beckman DxC, which may be methodological and of no clinical importance. The slightly low hypoproteinaemia flagging observed was probably due to rounding of the 62 g/L lower cut-off to 60 g/L for convenience and the flagging of calculated globulins may be a better approach to picking up immune deficiencies.
The high flagging rate for hyperkalaemia was generally close to the expected 2.5% background prevalence; however some laboratories had significantly higher levels. This was probably mainly due to pre-analytical issues (e.g. transport/haemolysis) as well as patient selection for the study. The private laboratories, including Sonic, were very sensitive to increasing the possibility of flag rates over 2.5% as this represented 1:40 urea and electrolyte requests that cause anxiety for clinicians and patients in general practice. As the critical limit for after-hours telephoning is 6.0 mmol/L or higher, and laboratories may have difficulties in controlling sample collection and transport (when not performed in their own collection centres), private laboratories were very reluctant to drop their historical serum cut-off for potassium from 5.5 mmol/L. It should also be noted that the 5.2 mmol/L cut-off is appropriate for serum but a lower cut-off (4.8–4.9 mmol/L) may be more appropriate for plasma samples due to the absence of potassium release from platelets and white cell disruption. High bicarbonates were extremely uncommon on Abbott and, as previously discussed, this should improve with the manufacturer’s recalibration. The low flag rates observed in private laboratories for hypercalcaemia mainly occurred in men and premenopausal women. Higher flag rates (3.2%) were observed for postmenopausal women, because a slight increase in bicarbonate at menopause increases total calcium, meaning that the 2.60 mmol/L cut-off is probably especially appropriate to this important group at risk of hyperparathyroidism. LDH results were generally more commonly elevated than 2.5%, and this may be because the ‘rounded’ 250 U/L cut-off may be slightly low. The clinical question is ‘how are LDH levels relied on for picking up early disease (e.g. haematological)?’ High alkaline phosphatase (ALP) levels using the 110 U/L cut-off were more common than 2.5% (about 8.5% with no real platform dependence). There are small differences in ALP between men and women, even following menopause, but these are hard to differentiate due to analytical issues i.e. 5 U/L vs 10 U/L may not be discernible. It was decided that an upper reference limit of 110 U/L was clinically important in detecting pathology in postmenopausal women.
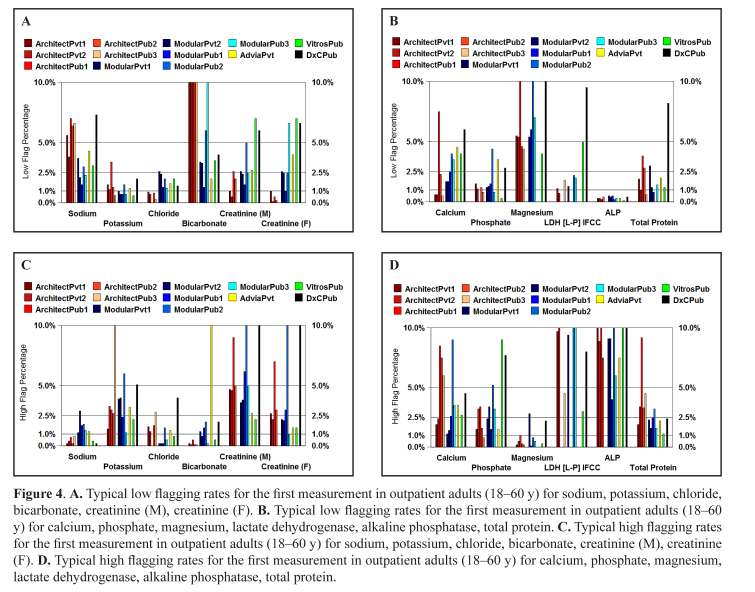
The low and high flag rates were compared for various methods and both public and private pathology laboratories for outpatient adults (18–60 y) taking their first reading and are shown in Figures 4A–D. The flag rates for Ortho Vitros and Beckman DxC are generally higher but specifically: the high flag rate for low sodiums and low flag rate for high sodiums with the DxC is consistent with the low bias in the bias study; the high flag rate for low creatinine and low flag rate for high creatinine with the Vitros is consistent with the low bias in the bias study, however the high flag rate for both high and low creatinine with the DxC is probably due to population issues; and the low flag rate for low phosphate and high flag rate for high phosphate with both Vitros and DxC are consistent with the high bias for these assays. Note that the flagging rates shown in Figures 4A–D are higher than the typical rates shown in Table 5 as outlier high flagging rates due to method biases and population issues have been included.
Figure 4.
A. Typical low flagging rates for the first measurement in outpatient adults (18–60 y) for sodium, potassium, chloride, bicarbonate, creatinine (M), creatinine (F). B. Typical low flagging rates for the first measurement in outpatient adults (18–60 y) for calcium, phosphate, magnesium, lactate dehydrogenase, alkaline phosphatase, total protein. C. Typical high flagging rates for the first measurement in outpatient adults (18–60 y) for sodium, potassium, chloride, bicarbonate, creatinine (M), creatinine (F). D. Typical high flagging rates for the first measurement in outpatient adults (18–60 y) for calcium, phosphate, magnesium, lactate dehydrogenase, alkaline phosphatase, total protein.
Final Selection of Common RI
The final decision on the common RI to be used involved weighing up each piece of evidence. First the bias study using commutable samples was assessed for clinical acceptability across the eight major platforms in use in Australasia based on the RCPAQAP allowable limits of performance. Next RI data from the Aussie Normals study were compared with other direct and indirect RI studies. Data mining from private pathology supported the Aussie Normals data while the paediatric RIs were based on data mining and consensus agreement across 31 paediatric laboratories within Australasia. Flagging rates were assessed to determine if a change to historical RIs such as the higher upper reference limit for potassium used in private pathology would create higher flag rates. Indeed, laboratory responses indicate that the pre-analytical effect of delayed sample transport would impact on potassium levels and hence for pragmatic reasons private pathology has chosen to have a RI of 3.5–5.5 mmol/L (Appendix 2). In the case of ALP RIs in postmenopausal women, some laboratories chose to use a higher upper reference limit (Appendix 2). Further information about the final selection of a common RI for each analyte decided at the 2014 AACB Harmonisation workshop is available at the AACB harmonisation website.5,20
Laboratory Acceptance Sought
In 2013 laboratories were invited to respond to the proposed common RIs. Either they were using the RI already, would accept the RI, or provided comments and their reason for not accepting the common RI. Within Australia 24 responses were received and represented all states, public and private pathology, small and large laboratories and networks. There was full acceptance of RIs for sodium, chloride and creatinine by Australian laboratories, >90% acceptance for bicarbonate, calcium, phosphate, LDH and total protein, and fewer agreed for potassium, magnesium and ALP (Appendix 2). This indicated there was likely to be a high adoption rate for the first panel of 11 common RIs.
5. Adoption Process and Principles
Validation
Responsibility for adoption of the common RIs still lies with each laboratory. Key questions are: ‘Is this RI suitable for my method and for my population?’ Validations of RIs may be by subjective assessment assuming the same method and the same population are used or by a simple validation using 20 normal subjects representing your population.21 Alternatively, the median of a data extract of outpatient results can be compared with the centre of the distributions used to set the common RI or, at a more complex level, Bhattacharya analysis can be used to assess the proposed intervals.22 If the local validation is not suitable then consider pre-analytical and analytical issues or a population difference.
Documentation
From the outset of the harmonised RIs project the AACB has tried to keep the membership informed of progress. All talks and breakout discussions were recorded as PowerPoint presentations and are displayed on the AACB website under the Harmonisation section.23 The harmonised checklist data have been faithfully recorded in a structured spreadsheet format which is also available on the AACB website. Apart from the RI data, the spreadsheet includes each manufacturer’s method and traceability information which is a useful resource for laboratories. Laboratories should document the source of their laboratory RIs in their laboratory manual. If the AACB common RIs are adopted then it is appropriate to state the source as ‘According to AACB Harmonised Reference Intervals project’, and to provide evidence of RI suitability through the RCPAQAP.
Conclusions
Consideration should be given by laboratories to adopting RIs consistent with those used by other laboratories in the region where it is possible and appropriate for the local population. Following an intensive scrutiny of the evidence supporting the harmonisation of RIs within Australasia, the profession has supported the concept and both the AACB and the RCPA have formally endorsed common RIs for use in Australia and New Zealand. The Australian National Association of Testing Authorities (NATA) recognises the ‘AACB Harmonised Reference Intervals’ as a reference source and recommends laboratories use these intervals and if not, to provide supporting evidence for other references. We strongly encourage laboratories to support this evidence-based initiative and to document and implement ‘AACB Harmonised Reference Intervals’.
Acknowledgments
The AACB thanks the following working groups and organisations for their participation and contribution to the Harmonisation workshops and to the common RIs project: AACB Common Reference Intervals Working Party, AACB Paediatric Biochemistry Working Party, AACB Harmonisation Committee, RCPA PITUS WG4, RCPAAACB Working Group on Critical Results Management, Sonic Health, ACT Pathology, Auckland Regional Quality Assurance Group, New Zealand South Island Quality Assurance Group, Alfred Pathology, Pathwest. Our thanks also go to Andrew St John, Peter Vervaart, Tony Badrick, Rita Horvath, Que Lam, David Kanowski, Robert Flatman, Ronda Greaves, Kristina Barancek, Narelle Hadlow, Andy Griffin, Michael Legg, Donna Moore, Lisa King, Erin McLemon, and importantly, to all participating delegates who attended the harmonisation workshops for their contributions. Finally, the AACB thanks the Australian Government’s Department of Health for financial support of the Harmonisation project through a QUPP grant.
Appendix 1. AACB Common Reference Intervals (RIs) implementation plan.
| Sequence of events | Adult common RIs (1st group) | Paediatric common RIs |
|---|---|---|
| Identify problem | Lack of a nationally co-ordinated review of common RIs for routine pathology analytes for both adult and paediatric populations in Australasia. | |
| Agree to address | Pathologists and Medical Scientists agreed to address common RIs at the same time as the RCPA PUTS and PITUS initiatives for standardisation of pathology units, terminology, and report formatting and flagging. | |
| Identify relevant groups | In 2011 AACB formed a Harmonisation Committee consisting of both Chemical Pathologists and Medical Scientists from large public and private pathology networks to address various harmonisation issues including common RIs, management of critical laboratory results, biochemistry units and terminology, test panels, etc. | |
| Seek formal co-operation (if external bodies involved) | AACB wrote to RCPA as well as to In Vitro Diagnostics Industry inviting their input into the Harmonisation Common RIs initiative. | |
| Form working group | In 2012 the AACB Common RIs working party was revamped to include Chemical Pathologists, Medical Scientists, and RCPAQAP and industry representatives. | In 2012 the AACB Paediatric Biochemistry working party was formed and consisted of Chemical Pathologists from the 9 major paediatric laboratories in Australia and New Zealand. |
| Describe problem in detail | The aim was to derive and validate common RIs through an evidence-based approach and extensive data analysis. | The aim was to survey paediatric RIs in use across Australasia and through a data mining approach to develop RIs covering birth to adulthood. |
| Allocate a budget and determine sources of funding | Funding was provided by AACB, by Harmonisation workshops, and through an Australian Government’s Department of Health Quality Use of Pathology Project grant of A$60,000. | |
| Gather information (surveys, RI studies, data mining, bias study, calibration traceability, RI verification laboratory information, flagging rates) | AACB Common RIs working party gathered evidence from the following sources:
|
An extensive data mining exercise was conducted of over 1.8 million results received from 15 paediatric laboratories. |
| Consider solutions | In May 2012 preliminary data from the Aussie Normals, bias and Sonic RI studies were reviewed and an initial set of adult common RIs developed at a 1-day meeting by the AACB Common RIs working party. Further data analysis was requested for some of the more difficult analytes; work continues for a second group of chemistry analytes. | Results of the Bhattacharya analysis were reviewed by the AACB Paediatric Biochemistry working party. |
| Produce discussion paper, etc. | Preliminary information about harmonised RIs was disseminated through various publications in the AACB Clinical Biochemist Newsletter and 2012 harmonisation issues of the Clinical Biochemist Reviews, and by various lectures and posters including those given by Dr Ken Sikaris in the 2012 Current Concepts tour, at RCPA Pathology Update conference and at the 2012 and 2013 AACB Annual Scientific Conferences. harmonisation of RIs within Australasia. | |
| Seek feedback from stakeholders | Representatives from all of the major laboratory networks together with invited clinicians and industry attended a series of three Harmonisation workshops convened by AACB in May 2012, July 2013 and April 2014 to discuss the evidence supporting common RIs. In late 2013 an Adult common RIs adoption spreadsheet was developed and sent to laboratories requesting confirmation of their intent to adopt the proposed general chemistry RIs. |
|
| Revise recommendations | AACB Common RIs working party met several times in 2013 and 2014 to review additional data and to plan for the workshops. | AACB Paediatric Biochemistry working party met before the workshops to discuss the paediatric RI data. |
| Obtain formal endorsement | The first group of Adult and Paediatric common RIs was ratified at the 2014 workshop by the 55 delegates attending the meeting. Formal endorsement was received from the AACB Executive, the RCPA Chemical Pathology Advisory Committee and RCPA Board. |
|
| Publish | The tables of common RIs will be available through the RCPA website. Information about the common RIs is published in this issue of The Clinical Biochemist Reviews and more details are available on the AACB website at: http://www.aacb.asn.au/professionaldevelopment/harmonisation. |
|
| Promote | AACB and RCPA will continue to promote and update common RIs through their working parties. | |
| Monitor introduction | RCPAQAP Liquid Serum Chemistry program provides a mechanism to audit the uptake of common RIs by laboratories. Future harmonisation workshops are planned to develop common RIs for other chemistry and endocrine analytes. |
|
Appendix 2. Adoption of common reference intervals by laboratories within Australia and New Zealand.
| Laboratory | State | Analyser | Analyte, Interval, Units | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Sodium 135–145 mmol/L |
Potassium 3.5–5.2 mmol/L |
Chloride 95–110 mmol/L |
Bicarbonate 22–32 mmol/L |
Creatinine (M)
60–110 umol/L |
Creatinine (F)
45–90 umol/L |
Calcium 2.10–2.60 mmol/L |
Phosphate 0.75–1.50 mmol/L |
Magnesium 0.70–1.10 mmol/L |
LDH [L to P] IFCC 120–250 U/L |
ALP 30–110 U/L |
Total Protein 60–80 g/L |
|||
| AUST #1 | NSW | Roche Modular | A | A | C | A | A | A | C | A | N | A | A | A |
|
| ||||||||||||||
| AUST #2 | QLD | Beckman-Coulter DxC series | C | A | A | C | A | A | A | A | C | A* | N | A |
|
| ||||||||||||||
| AUST #3 | VIC | Not indicated | A | A | A | A | A | A | A | A | A | A | A | A |
|
| ||||||||||||||
| AUST #4 | QLD | Ortho Vitros | C | A | A | A | C | C | A | N | A | A | A | N |
|
| ||||||||||||||
| AUST #5 | TAS | Abbott Architect | C | A | C | A | A | A | A | A | A | A | A | C |
|
| ||||||||||||||
| AUST #6 | TAS | Abbott Architect | A | A | A | A | A | A | C | A | A | A | A | A |
|
| ||||||||||||||
| AUST #7 | NSW | Abbott and Roche | C | N 3.5–5.5 |
C | P† | C‡ | C | A | C | C | C | A§ | A |
|
| ||||||||||||||
| AUST #8 | QLD | Abbott and Roche | C | N 3.5–5.5 |
C | P† | C‡ | C | A | C | C | C | A§ | A |
|
| ||||||||||||||
| AUST #9 | VIC | Roche | C | N 3.5–5.5 |
C | C | C‡ | C | A | C | C | C | A§ | A |
|
| ||||||||||||||
| AUST #10 | ACT | Roche | C | N 3.5–5.5 |
C | C | C‡ | C | A | C | C | C | A§ | A |
|
| ||||||||||||||
| AUST #11 | SA | Roche | C | N 3.5–5.5 |
C | C | C‡ | C | A | C | C | C | A§ | A |
|
| ||||||||||||||
| AUST #12 | WA | Roche | C | N 3.5–5.5 |
C | C | C‡ | C | A | C | C | C | A§ | A |
|
| ||||||||||||||
| AUST #13 | TAS | Roche | C | N 3.5–5.5 |
C | C | C‡ | C | A | C | C | C | A§ | A |
|
| ||||||||||||||
| AUST #14 | NSW | Roche Cobas | C | N | C | C | C | A | A | C | C | C | C | C |
|
| ||||||||||||||
| AUST #15 | NSW | Abbott Architect | A | A | A | A | A | A | A | A | A | A | A | A |
|
| ||||||||||||||
| AUST #16 | NSW | Abbott Architect | A | A | A | A | A | C | A | A | A | A | A | A |
|
| ||||||||||||||
| AUST #17 | ACT | Abbott Architect | A | A | A | A | C | A | A | A | A | A | A | C |
|
| ||||||||||||||
| AUST #18 | WA | Abbott Architect and Ortho Vitros | A | A | A | C | C | C | A | A | C | A | A | C |
|
| ||||||||||||||
| AUST #19 | SA | Siemens Advia, Roche and Beckman-Coulter | A | A | A | C | A | A | A | A | A | A | C | A |
|
| ||||||||||||||
| AUST #20 | NSW | Roche Cobas Integra 6000/8000 | C | A | A | C | C | A | A | A | A | A | A | A |
|
| ||||||||||||||
| AUST #21 | VIC | Not indicated | C | A | A | C | A | A | A | A | A | A | N 8.7% above 110 U/L |
C |
|
| ||||||||||||||
| AUST #22 | VIC | Siemens Advia and Roche Cobas Integra | A | A | C | A | C | A | A | A | N | N | A | |
|
| ||||||||||||||
| AUST #23 | NSW | Siemens Advia 2400 | C | N 3.6–5.4 |
A | A | A | A | A | A | C | A | N | N |
|
| ||||||||||||||
| AUST #24 | NZ | Not indicated | C | C | C | N 22–29 |
C | C | N 2.15–2.55 |
N 0.8–1.5 |
C | N | ||
|
| ||||||||||||||
| NZ #1 | NZ | Not indicated | C | C | C | N 22–29 |
C | C | N 2.15–2.55 |
N 0.8–1.5 |
C | N P to L |
N 20–110 |
N 65–80 |
|
| ||||||||||||||
| NZ #2 | NZ | Not indicated | C | C | C | A | A | C | A | C | A | A | A | A |
|
| ||||||||||||||
| NZ #3 | NZ | Not indicated | A | A | A | A | A | A | N 2.15–2.55 |
A | A | N P to L |
N 20–110 |
N 65–80 |
|
| ||||||||||||||
| NZ #4 | NZ | Not indicated | C | C | Not applicable | Not applicable | N 50–110 |
A | N 2.15–2.55 |
N 0.75–1.55 |
Not applicable | N 110–220 |
N 40–130 over 60y M & F |
N 60–87 |
|
| ||||||||||||||
| NZ #5 | NZ | Not indicated | C | C | C | A | A | C | A | C | A | A | A | A |
|
| ||||||||||||||
| NZ #6 | NZ | Not indicated | C | C | C | N 22–31 |
N 60–105 |
C | C | C | N 0.7–1.0 |
C | N 40–110 |
N 66–84 |
|
| ||||||||||||||
| Summed A and C for Australian Pathology organizations | 24 | 15 | 24 | 21 | 24 | 24 | 23 | 22 | 22 | 23 | 19 (includes §labs) | 21 | ||
AUST, Australia; NZ, New Zealand; A, laboratory agrees to ACCEPT these common reference intervals following official endorsement; C, reference intervals are CURRENTLY in use; N, laboratory has evaluated the intervals and found them NOT suitable for use; P, laboratory is planning to introduce these intervals irrespective of official endorsement; LDH: L to P, lactate dehydrogenase: lactate to pyruvate.
LDH requires consultation with haematologists.
Bicarbonate – pending Architect new assay validation 22–32 mmol/L.
Creatinine – laboratory can choose to use age-related RIs at >60 y.
Alkaline phosphatase (ALP) – applicable for adult males and premenopausal females only
Footnotes
Competing Interests: None declared (JT, GK, MR, JG, GK, PG, JR) GRDJ has received research support from Roche and honoraria from Bio-Rad, Roche and Abbott Diagnostics.
References
- 1.Rustad P, Felding P, Franzson L, Kairisto V, Lahti A, Mårtensson A, et al. The Nordic Reference Interval Project 2000: recommended reference intervals for 25 common biochemical properties. Scand J Clin Lab Invest. 2004;64:271–84. doi: 10.1080/00365510410006324. [DOI] [PubMed] [Google Scholar]
- 2.Berg J, Lane V. Pathology Harmony; a pragmatic and scientific approach to unfounded variation in the clinical laboratory. Ann Clin Biochem. 2011;48:195–7. doi: 10.1258/acb.2011.011078. [DOI] [PubMed] [Google Scholar]
- 3.Jones GR, Barker A, Tate J, Lim C-F, Robertson K. The case for common reference intervals. Clin Biochem Rev. 2004;25:99–104. [PMC free article] [PubMed] [Google Scholar]
- 4.Survey of laboratory reference intervals conducted in 2010. http://www.aacb.asn.au/documents/item/379 (Accessed 4 September 2014).
- 5.CRI – validation and implementation of a first panel. http://www.aacb.asn.au/documents/item/2791 (Accessed 4 September 2014).
- 6.RCPA PUTS Project (Chemical Pathology Working Group) http://www.rcpa.edu.au/getattachment/fa16479c-7474-4fd9-b947-8c6d270588c7/APUTS-Chemical-Pathology-Reporting-Terminology-ref.aspx (Accessed 6 June 2014).
- 7.Australian standards for electronic messaging and representation of Pathology results, units and flagging including AS4700.2 and HB262. http://www.e-health.standards.org.au/Home/Publications.aspx (Accessed 6 June 2014).
- 8.Sikaris KA. Physiology and its importance for reference intervals. Clin Biochem Rev. 2014;35:3–14. [PMC free article] [PubMed] [Google Scholar]
- 9.Jones G, Barker A. Reference intervals. Clin Biochem Rev. 2008;29(Suppl 1):S93–7. [PMC free article] [PubMed] [Google Scholar]
- 10.KDIGO. 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Chapter 1. Kidney Int Suppl. 2013;3:19–62. doi: 10.1038/ki.2013.243. http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf (Accessed 6 June 2014). [DOI] [PubMed] [Google Scholar]
- 11.Doumas BT, Bayse DD, Carter RJ, Peters T, Jr, Schaffer R. A candidate reference method for determination of total protein in serum. I. Development and validation. Clin Chem. 1981;27:1642–50. [PubMed] [Google Scholar]
- 12.Koerbin G, Sikaris KA, Jones GR, Ryan J, Reed M, Tate J, AACB Committee for Common Reference Intervals Evidence-based approach to harmonised reference intervals. Clin Chim Acta. 2014;432:99–107. doi: 10.1016/j.cca.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Jones GR, Sikaris K, Gill J. ‘Allowable Limits of Performance’ for External Quality Assurance Programs - an Approach to Application of the Stockholm Criteria by the RCPA Quality Assurance Programs. Clin Biochem Rev. 2012;33:133–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Reference Methods and Traceability. http://www.aacb.asn.au/professionaldevelopment/methods/reference-methods-and-traceability (Accessed 4 September 2014).
- 15.JCTLM. Joint Committee for Traceability in Laboratory Medicine. http://www.bipm.org/jctlm/ (Accessed 6 June 2014).
- 16.Ichihara K, Itoh Y, Lam CW, Poon PM, Kim JH, Kyono H, et al. Science Committee for the Asian-Pacific Federation of Clinical Biochemistry Sources of variation of commonly measured serum analytes in 6 Asian cities and consideration of common reference intervals. Clin Chem. 2008;54:356–65. doi: 10.1373/clinchem.2007.091843. [DOI] [PubMed] [Google Scholar]
- 17.Badrick T, Hickman P. Significant figures. Clin Biochem Rev. 2008;29(Suppl i):S89–91. [PMC free article] [PubMed] [Google Scholar]
- 18.Badrick T, Hawkins RC. The relationship between measurement uncertainty and reporting interval. Ann Clin Biochem. 2014 doi: 10.1177/0004563214531558. [DOI] [PubMed] [Google Scholar]
- 19.Ayuk J, Gittoes NJ. Contemporary view of the clinical relevance of magnesium homeostasis. Ann Clin Biochem. 2014;51:179–88. doi: 10.1177/0004563213517628. [DOI] [PubMed] [Google Scholar]
- 20.Impact of proposed HRI’s on laboratory report flagging rates. http://www.aacb.asn.au/documents/item/2796 (Accessed 4 September 2014).
- 21.Clinical and Laboratory Standards Institute. How to define and determine reference intervals in the clinical laboratory; approved guideline. third edition. Wayne, PA: CLSI; 2008. CLSI document C28-A3. [Google Scholar]
- 22.Jones GR. Validating common reference intervals in routine laboratories. Clin Chim Acta. 2014;432:119–21. doi: 10.1016/j.cca.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Harmonisation – Australasian Association of Clinial Biochemists. http://www.aacb.asn.au/professionaldevelopment/harmonisation (Accessed 4 September 2014).
- 24.Guidelines for the assessment of absolute cardiovascular disease risk. http://www.heartfoundation.org.au/SiteCollectionDocuments/guidelines-Absolute-risk.pdf (Accessed 8 September 2014).
- 25.Workshop appendix report by analyte. http://www.aacb.asn.au/documents/item/351 (Accessed 4 September, 2014).