Abstract
Background. Liver diseases still represent a major health burden worldwide. Moreover, medicinal plants have gained popularity in the treatment of several diseases including liver. Thus, the present study was to evaluate the effectiveness of Piper cubeba fruits in the amelioration of CCl4-induced liver injuries and oxidative damage in the rodent model. Methods. Hepatoprotective activity was assessed using various biochemical parameters like SGOT, SGPT, γ-GGT, ALP, total bilirubin, LDH, and total protein. Meanwhile, in vivo antioxidant activities as LPO, NP-SH, and CAT were measured in rat liver as well as mRNA expression of cytokines such as TNFα, IL-6, and IL-10 and stress related genes iNOS and HO-1 were determined by RT-PCR. The extent of liver damage was also analyzed through histopathological observations. Results. Treatment with PCEE significantly and dose dependently prevented drug induced increase in serum levels of hepatic enzymes. Furthermore, PCEE significantly reduced the lipid peroxidation in the liver tissue and restored activities of defense antioxidant enzymes NP-SH and CAT towards normal levels. The administration of PCEE significantly downregulated the CCl4-induced proinflammatory cytokines TNFα and IL-6 mRNA expression in dose dependent manner, while it upregulated the IL-10 and induced hepatoprotective effect by downregulating mRNA expression of iNOS and HO-1 gene.
1. Introduction
A large number of crucial functions needed to regulate homeostasis such as detoxification and excretion are performed by liver; however liver diseases are still major health concerns in spite of the progress made in the field of medicine and pharmaceutical sciences. Numerous side effects are associated with synthetic drugs used in treating hepatic disorders. Consequently traditional herbal drugs, spices, fruits, vegetables, and medicinal plants have gained popularity over the past decades owing to their safety and efficacy.
The fruits of Piper cubeba L. (Piperaceae) are commonly known as cubeba in Arabic and tailed piper in English. They are spices and possess various medicinal properties [1]. In traditional medicine they are used as stimulants, appetizers, stomachics, and expectorants. The fruit of cubeba is also used to relive the gastric pain, enteritis, diarrhea, and anti-inflammatory agent. Furthermore, cubeba fruits are known to possess pain and inflammation reducing capacity in experimental animals [2], which is attributed to the antioxidant activity of some isolated chemical constituents [3]. The fruits are also reported to possess antibacterial, antifungal, bactericidal (Helicobacter pylori), and renal protective properties [4–6]. P. cubeba is used by the traditional medicine practitioner in treating acute jaundice. In an earlier study ethanolic extract of P. cubeba has been shown to enhance the activity of pioglitazone and act synergistically in lowering the blood glucose level in rats [7]. There are several lines of evidence which implicated oxidative stress and inflammation in the etiology of liver diseases, cardiovascular diseases, and cancer [8, 9]. As a result, carbon tetrachloride (CCl4), which produces reactive free radicals when metabolized, has been widely used as a solvent for induction of hepatic damage in animal models [10]. CCl4 increases lipid peroxidation in hepatic cells and induces liver damage and necrosis [11]. To the best of our knowledge there was a lack of scientific reports available in support of its traditional claim of hepatoprotective potential. So far, there has been no research reported on hepatoprotective effect against carbon tetrachloride induced liver damage in rats. Hence, the present study was aimed at investigating the possible potential hepatoprotective effects of the Piper cubeba ethanolic extract (PCEE) against CCl4-induced hepatic injuries in male Wistar rats.
2. Material and Methods
2.1. Animals
Healthy male Wistar albino rats (180–200 g) were used for the study. Animals were issued from Central Animal House Facility of King Saud University and were kept in standard plastic animal cages in groups of 6 animals each with 12-hour light and dark cycle at 25 ± 2°C. The rats were fed on standard rat chow and provided water ad libitum. The animals were acclimatized to laboratory conditions for a week prior to experiments.
2.2. Plant Material and Preparation of Extract
The fruits of Piper cubeba were purchased from the local vegetable market in Riyadh and their identity was confirmed at the Department of Pharmacognosy, College of Pharmacy, King Saud University. The dried fruits (500 g) were coarsely powdered and macerated in 3 L of 70% ethanol for 72 h using percolation method. The solvent was then removed at 40°C under reduced pressure in a rotatory evaporator. The Piper cubeba ethanolic extract (PCEE) was then suspended in distilled water just before its administration to the animals.
2.3. Acute Toxicity
Male Wistar albino rats were divided into test groups comprising six animals in each group. The test was performed using increasing oral dose of herbal extract from 100 to 1000 mg/kg body weight. The rats were observed continuously for 1 h and then half hourly for 4 h for any gross behavioral change and general motor activities like writhing, convulsion, response to tail pinching, gnawing, pupil size, fecal output, feeding behavior, and so forth and further up to 72 h for any mortality. The extract does not cause any significant behavioral changes and no mortality was observed.
2.4. Experimental Procedure
Rats were randomly divided into five groups of six animals each. Group I received normal saline for 7 days and served as normal control. Group II received normal saline (1 mL/kg, p.o.) for 7 days and served as toxic control. Groups III and IV were prophylactically treated with plant extract at a dose of 250 and 500 mg/kg p.o. each, respectively. Group V served as positive control and was prophylactically treated with silymarin (10 mg/kg, p.o.) for 7 days. On the 8th day, Groups II, III, and IV and Group V were injected intraperitoneally (i.p.) with 0.4 mL/kg CCl4 as a 20% solution in paraffin oil, while the other two groups were given an equal amount of paraffin oil. After 24 h of CCl4-induced hepatotoxicity, the blood was collected from retro-orbital plexus under light ether anesthesia in tubes containing disodium EDTA. Plasma was separated by centrifugation at 2500 ×g for 10 min and was transferred to prelabeled eppendrof tubes for various biochemical parameters. Immediately, after blood withdrawal the animals were sacrificed and liver samples were collected for histopathological and biochemical estimations. Liver samples were washed with chilled normal saline, and 10% (w/v) liver homogenates were prepared in ice cold 0.15 M KCl solution using motor driven Teflon pestle. The animals were approved by Institutional Animal Ethics Committee of College of Pharmacy, King Saud University, Riyadh, Saudi Arabia.
2.5. Biochemical Estimation
Different biochemical parameters like serum glutamic oxaloacetic transaminase (SGOT) [12], serum glutamic pyruvic transaminase (SGPT) [13], Υ-glutamyl transpeptidase (GGT) [14], alkaline phosphatase [14], total bilirubin [15], and lactic acid dehydrogenase (LDH) [16], respectively, were estimated in serum.
2.6. Estimation of Malondialdehyde (MDA) in Hepatic Tissue
The method reported by Utely et al. [17] was followed. The animals were killed 1 h after ethanol administration. The liver tissues were removed and homogenized in 0.15 mol/L KCl (at 4°C) in a Potter-Elvehjem type C homogenizer to give a 10% w/v homogenate. Aliquots of homogenate 1 mL in volume were incubated at 37°C for 3 h in a metabolic shaker. Then 1 mL of 10% aqueous TCA was added and mixed. The mixture was then centrifuged at 800 ×g for 10 min. One milliliter of the supernatant was removed and mixed with 1 mL of 0.67% 2-thiobarbituric acid in water and placed in a boiling water bath for 10 min. The mixture was cooled and diluted with 1 mL distilled water. The resulting chromogen absorbance was determined at the wavelength of 532 nm at room temperature against blank reference. The concentration of MDA was read from standard calibration curve plotted using 1,1,3,3'-tetraethoxypropane (TEP). The extent of lipid peroxidation was expressed as MDA values are expressed as nanomoles of MDA per gram of protein using a molar extinction coefficient for MDA of 1.56 × 105 M−1 cm−1 [18]. The protein content was estimated according to the method of Lowry et al. (1951) [19].
2.7. Estimation of Nonprotein Sulfhydryl (NP-SH) in Hepatic Tissue
Hepatic nonprotein sulfhydryls were measured according to the method of Sedlak and Lindsay (1968) [20]. The hepatic tissue was homogenized in ice-cold normal saline containing 0.02 mmol/L ethylenediaminetetraacetic acid (EDTA). Aliquots of 5 mL of the homogenates were mixed in 15 mL test tubes with 4 mL of distilled water and 1 mL of 50% trichloroacetic acid (TCA). The tubes were shaken intermittently for 10 min and centrifuged at 3000 rpm/min. Two milliliters of supernatant was mixed with 4 mL of 0.4 mol/L Tris buffer at pH 8.9. 0.1 mL of 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB) was added and the sample was shaken. The absorbance was measured within 5 min of DTNB addition at 412 nm against a reagent blank.
2.8. Estimation of Catalase Activity and Total Protein in Hepatic Tissue
Catalase activity was measured according to the method described by Aebi (1974) [21]. Supernatant (0.1 mL) was added to cuvette containing 1.9 mL of 50 mM phosphate buffer (pH 7.0). Reaction was started by the addition of 1.0 mL of freshly prepared 30 mM H2O2. The rate of decomposition of H2O2 was measured spectrophotometrically from changes in absorbance at 240 nm. Activity of catalase was expressed as U/mg protein. The total protein was estimated in liver homogenate and determined spectrophotometrically by using Folin phenol reagent [19].
2.9. Total RNA Isolation
Twenty-four hours following CCl4 administration, the liver samples were obtained to be used for total RNA isolation according to the manufacturer's instructions of the Trizol reagent (Life Technologies, Inc., Grand Island, NY, USA). Following total RNA isolation, the reverse transcription from total RNA to cDNA was processed by high capacity cDNA reverse transcription kit of Applied Biosystems according to the manufacturer's instructions [22]. Concisely, RNA was diluted to 2 × RT master mix buffer; 2 μL dNTP (10 mM), 1 μL RNase inhibitor, and 1 μL hexamer primers were added to 10 μL of diluted RNA on ice. The mixture was heated at 65°C for 10 min and then snap-cooled on ice for 2 min, prior to the addition of 2 μL of reverse transcriptase (200 units μL−1). The reaction was carried out at 25°C for 10 min then 37°C for 120 min fallowed by 72°C for 4 minutes. The cDNA was stored at −80°C until further use.
2.10. Expression of TNF-α, IL-10, HO-1, and iNOS-2 mRNA in Hepatic Tissue
Quantitative analysis of specific mRNA expression was performed by RT-PCR by subjecting the resulting cDNA to PCR amplification using 96-well optical reaction plates in the ABI Prism 7500 System (Applied Biosystems). The 25 μL reaction mixture contained 0.1 μL of 10 μM forward primer and 0.1 μL of 10 μM reverse primer (40 nM final concentration of each primer), 12.5 μL of SYBR Green Universal Master mix, 11.05 μL of nuclease-free water, and 1.25 μL of cDNA sample. Rat primers TNF-α, IL-10, HO-1, iNOS-2, and β-ACTIN gene were synthesized and purchased from Integrated DNA Technologies (IDT, Coralville, IA) (Table 1). The fold change in the level of mRNA between treated and untreated groups was corrected by the levels of β-ACTIN. The RT-PCR data were analyzed using the relative gene expression, that is, (DD CT) method, as described and explained previously [23, 24]. Briefly, the data are presented as the fold change in gene expression normalized to the endogenous reference gene β-ACTIN and relative to a calibrator. The fold change in the level of target genes between treated and untreated groups, corrected by the level of β-ACTIN, was determined using the following equation: fold change = 2−Δ(ΔCt), where ΔCt = Ct(target) − Ct(β-actin) and Δ(ΔCt) = ΔCt(treated) − ΔCt(untreated). All reactions were run in duplicate. The hot start polymerase was activated by heating at 95°C for 3 min. The cycling conditions were 0.1 min at 95°C (melting) and 0.45 min at 60°C (annealing and extension). Threshold values (Ct) were calculated automatically by the software. The Ct data was processed according to the method described by Pfaffl briefly [24]; the Pfaffl equation was first used to calculate the relative gene expression ratio, that is, the change in target gene expression divided by the change in the reference gene expression.
Table 1.
List of primers.
| Gene | Primer | Reference |
|---|---|---|
| TNF-α | F-GTAGCCCACGTCGTAGCAAA-3′ | [26] |
| R-5′-CCCTTCTCCAGCTGGAAGAC-3 | ||
|
| ||
| IL-6 | F-5-CTTCCAGCCAGTTGCCTTCT | [27] |
| GACAG CATTGGAAGTTGGGG | ||
|
| ||
| IL-10 | F-5-GGAGTGAAGACCAAAGG-3′ | [26] |
| R-5′-TCTCCCAGGGAATTCAAATG-3′ | ||
|
| ||
| iNoS-2 | 5′-TTCTTTGCTTCTGTGCTTAATGCG-3 | [26] |
| 5′-GTTGTTGCTGAACTTCCAATCGT-3′ | ||
|
| ||
| HO-1 | 5′-CAGAAGGGTCAGGTGTC-3 | [28] |
| 5′-AGTAACTCCCACCTCGT-3′ | ||
|
| ||
| β-Actin | 5′-CCAGATCATGTTTGAGACCTTCAA-3′ | [29] |
| 5′-GTGGTACGACCAGAGGCATACA-3′ | ||
2.11. Histopathological Studies
Liver tissues were sliced in small pieces and immersed in neutral buffered 10% formalin for 24 h. The fixed tissues were processed routinely, embedded in paraffin (to get paraffin sections 4-5 μm), sectioned, deparaffinized, and rehydrated using the standard techniques (Bancroft and Gamble). The sections were then stained with Haematoxylin-Eosin dye and studied for histopathological changes [25].
3. Studies of the In Vitro Antioxidant Activity
3.1. Scavenging Activity of DPPH Radical
The radical scavenging ability of the PCEE against DPPH was evaluated as previously described [30]. In the presence of an antioxidant which can donate an electron to DPPH, the purple color, typical for free DPPH radical decays, and the change in absorbency at λ = 517 nm were measured. The test provides information on the ability of a compound to donate a hydrogen atom, on the number of electrons a given molecule can donate, and on the mechanism of antioxidant action. PCEE was redissolved in methanol and various concentrations (10, 50, 100, 500, and 1000 μg/mL) of the extract; 125 μL prepared DPPH (1 mM in methanol) and 375 μL solvent (methanol) were added. After 30 min incubation at 25°C, the decrease in absorbance was measured at λ = 517 nm. The radical scavenging activity was calculated from the following equation:
| (1) |
3.2. β-Carotene-Linoleic Acid Assay
The antioxidant activity of the extract was evaluated using the β-carotene bleaching method described and modified by Mothana (2011) [31]. One mL of a 0.2 mg/mL β-carotene solution in chloroform was added to flasks containing 0.02 mL of linoleic acid and 0.2 mL of Tween-20. The chloroform was removed at 40°C using a rotary evaporator. The resultant mixture was immediately diluted with 100 mL of distilled water and mixed for 1-2 min to form an emulsion. A mixture prepared similarly but without β-carotene was used as a blank. A control containing 0.2 mL of 80% (v/v) methanol instead of extract was also prepared. A 5 mL aliquot of the emulsion was added to a tube containing 0.2 mL of the sample extract at 1 mg/mL. Rutin (1 mg/mL) was used as a standard. The tubes were placed in a water bath at 40°C for 2 h. Absorbance was read at 470 nm at 15 min intervals. The antioxidant activity was calculated using the following equation:
| (2) |
where Abs0 and Abs0° are the absorbance values measured at zero time of incubation for sample extract and control, respectively. Abst and Abst° are the absorbance values for sample extract and control, respectively, at t = 120 min.
3.3. Phytochemical Screening
Preliminary phytochemical screening for terpenoids, alkaloids, flavonoids, anthraquinones, saponins, carbohydrates, tannins, and coumarins was performed with the extract by using chemical methods and thin-layer chromatography (TLC) according to the methodology described by Wagner and Bladt (1996) [32].
3.4. Statistical Data Analysis
Results are expressed as mean ± S.D. Total variation present in a set of data was estimated by one-way analysis of variance (ANOVA) followed by Dunnett's t-test. P < 0.01 was considered significant.
4. Results
4.1. Protective Effect of PCEE on CCl4-Induced Hepatotoxicity
Preliminary studies on Piper cubeba extract were devoid of any toxicity in rats when given in dose up to 1000 mg/kg by oral route. Hence, for further study 250 and 500 mg/kg doses of extract were selected. A significant increase in serum biomarkers such as serum SGOT, SGPT, GGT, ALP, total bilirubin, and LDH was observed in animals treated with CCl4, which is indicative of hepatic failure. PCEE at a dose of 250 and 500 mg/kg p.o. pretreatment for 7 days decreased the levels of abovementioned parameters significantly (P < 0.05, P < 0.01, and P < 0.001) in groups III and IV. Moreover, silymarin (group V) pretreatment produced highly significant decrease (P < 0.001) in serum SGOT, SGPT, GGT, ALP total bilirubin, and LDH. The amelioration of CCl4-induced hepatic injuries by PCEE is comparable with silymarin (Table 2).
Table 2.
Effect of Piper cubeba ethanolic extract on liver markers in CCl4-induced hepatotoxicity.
| Treatments | Dose mg/kg | SGOT (U/L) | SGPT (U/L) | GGT (U/L) | ALP (U/L) | Bilirubin (mg/dL) | LDH (U/L) |
|---|---|---|---|---|---|---|---|
| Normal | 87.28 ± 4.15 | 31.96 ± 3.05 | 4.90 ± 0.32 | 318.50 ± 6.34 | 0.52 ± 0.02 | 91.72 ± 3.81 | |
| CCl4 | 1.25 mL/kg | 222.33 ± 15.57∗∗∗a | 164.83 ± 9.08∗∗∗a | 16.18 ± 0.93∗∗∗a | 471.16 ± 12.03∗∗∗a | 2.36 ± 0.05∗∗∗a | 172.68 ± 8.08∗∗∗a |
| PCEE + CCl4 | 250 | 218.00 ± 6.91b | 166.16 ± 9.61b | 14.16 ± 0.41b | 410.00 ± 8.89∗∗b | 1.84 ± 0.08∗∗∗b | 160.34 ± 6.08b |
| PCEE + CCl4 | 500 | 154.00 ± 6.71∗∗b | 120.10 ± 8.41∗∗b | 12.05 ± 0.38∗∗b | 364.50 ± 12.20∗∗∗b | 1.53 ± 0.05∗∗∗b | 139.59 ± 4.21∗∗b |
| Silymarin + CCl4 | 10 | 137.83 ± 5.85∗∗∗b | 96.66 ± 5.99∗∗∗b | 9.33 ± 0.22∗∗b | 355.50 ± 13.97∗∗∗b | 0.92 ± 0.05∗∗∗b | 117.02 ± 3.65∗∗∗b |
All values represent mean ± SEM. ** P < 0.01; *** P < 0.001; a P < 0.05; b P < 0.01; c P < 0.001 ANOVA, followed by Dunnett's multiple comparison test.
∗,∗∗,∗∗∗Compared to normal group; a,b,ccompared to CCl4 only group.
4.2. Effect of PCEE on the Level of MDA
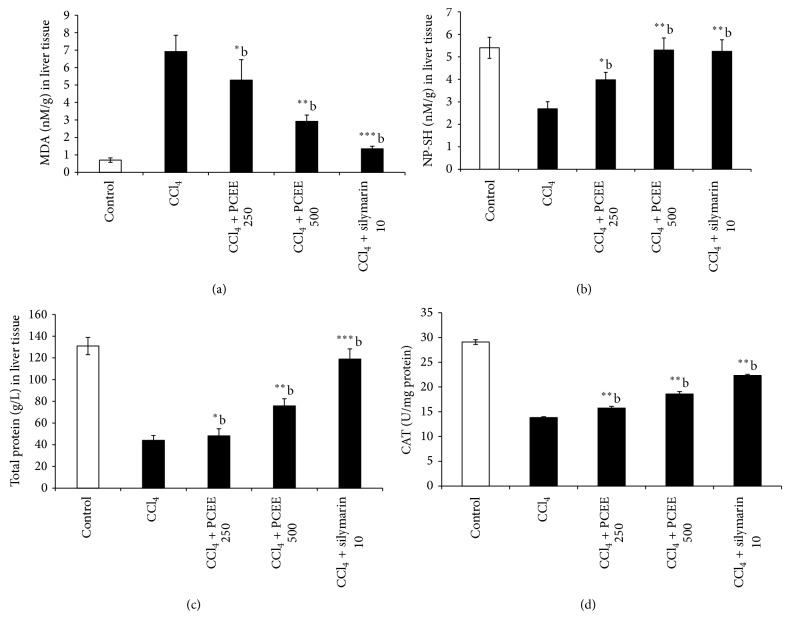
The extent of lipid peroxidation is measured by the formation of thiobarbituric acid reactive substances (TBARS). There is a sharp increase in (TBARS) level in CCl4-treated rats (group II) indicative of oxidative stress. The PCEE 250 and 500 mg/kg + CCl4-treated rats (groups III and IV) showed significant dose dependent reduction of TBARS level as compared to CCl4-treated rats (group II). This clearly showed reduction of oxidative stress by PCEE. Figure 1(a) showed clear significant change in the antioxidant levels of TBARS in CCl4 intoxicated rats as 6.92 ± 0.93 (P < 0.001) compared to control group. The change in TBARS level is comparable with silymarin.
Figure 1.
Effect of Piper cubeba ethanolic extract on (a) MDA, (b) NP-SH, (c) total protein level, and (d) catalase levels in the liver tissue of the rats treated with CCl4. All values represent mean ± SEM. ** P < 0.01; *** P < 0.001; a P < 0.05; b P < 0.01; c P < 0.001 ANOVA, followed by Dunnett's multiple comparison test. ∗,∗∗,∗∗∗Compared to normal group; a,b,ccompared to CCl4 only group.
4.3. Effect of PCEE on the Level of NP-SH
CCl4 intoxicated rats (group II) showed a significant decrease of NP-SH content indicative of an increase in protein metabolism whereas there was a dose dependent increase in NP-SH content significantly in groups III and IV (PCEE 250 and 500 mg/kg + CCl4). Silymarin pretreatment (group V) produced highly significant increase in NP-SH (P < 0.001). This clearly indicates that PCEE has ability to replenish the NP-SH content to normal levels (Figure 1(b)).
4.4. Effect of PCEE on the Level of Total Protein
Figure 1(c) showed a significant decrease of total protein content in CCl4 intoxicated rats which was indicative of hepatic injuries caused due to oxidative stress. Moreover, a dose dependent increase in total protein content was significantly observed in groups III and IV (PCEE 250 and 500 mg/kg + CCl4). Silymarin pretreatment (group V) produced highly significant increase in total proteins (P < 0.001). This clearly indicates that PCEE and silymarin have the ability to induce cell proliferation in hepatic tissue (Figure 1(c)).
4.5. Effect of PCEE on the Level of Catalase
CCl4 treatment caused a significant (P < 0.001) decrease in the level of catalase in liver tissue when compared with control group. The pretreatment with PCEE at both doses resulted in a significant increase of catalase level when compared to CCl4-treated rats. Silymarin showed also a significant increase in antioxidant enzymes levels compared to CCl4-treated rats (Figure 1(d)).
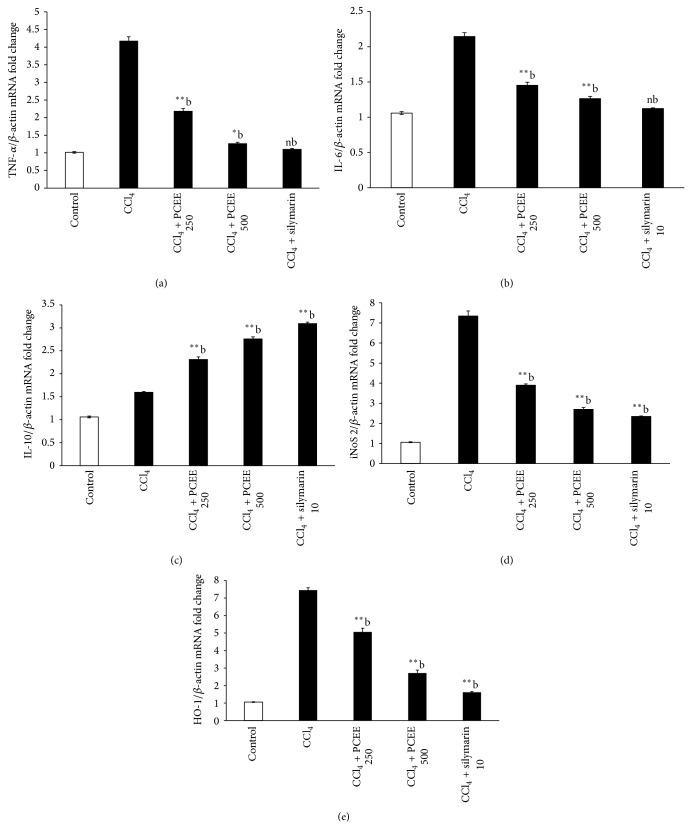
4.6. Effect of PCEE on mRNA Expression of Cytokines Genes Such as Tumor Necrotic Factor-α (TNF-α), Interleukin-6 (IL-6), and Interleukin-10 (IL-10)
The hepatic level of tumor necrotic factor-α (TNF-α) and interleukin-6 (IL-6) on mRNA expression in CCl4 intoxicated rats was approximately 4.5- and 2.1-fold higher than that in the control rats, as shown in Figures 2(a) and 2(b), while the PCEE + CCl4-treated rats showed significant dose dependent inhibition of TNF-α and mRNA expression as compared to CCl4 intoxicated rats. In addition, treatment of rats with PCEE for one week prior to CCl4 administration increased the mRNA expression of IL-10, as shown in Figure 2(c), which adds more evidence of the anti-inflammatory effect of PCEE in the acute phase response to CCl4. The upregulation of IL-10 by PCEE indicates its ability to downregulate the inflammatory cytokine. The hepatic level of iNOS-2 on mRNA expression in CCl4-treated rats was approximately 7.3-fold higher than that in the control rats. There is a dose dependent significant downregulation in rat groups previously treated with PCEE, silymarin + CCl4 (Figure 2(d)). This further indicates that PCEE and silymarin have anti-inflammatory activities.
Figure 2.
Effect of Piper cubeba ethanolic extract on mRNA expression of cytokines genes: (a) tumor necrotic factor-α (TNF-α), (b) interleukin-6 (IL-6), (c) interleukin-10 (IL-10), (d) inducible nitric oxide synthase gene (iNOS), and (e) inducible heamoxygenase (HO-1) gene. All values represent mean ± SEM. ** P < 0.01; *** P < 0.001; a P < 0.05; b P < 0.01; c P < 0.001 ANOVA, followed by Dunnett's multiple comparison test. ∗,∗∗,∗∗∗Compared to normal group; a,b,ccompared to CCl4 only group.
The extent of heme catabolism, as shown in Table 2, illustrated significantly higher levels of bilirubin in serum of CCl4-treated rats compared to that of the control group. The same trend is observed in the inducible HO-1 on mRNA expressions which were significantly increased 7.4-fold (P ≤ 0.01) in CCl4 group compared to control (Figure 2(e)). Conversely, control did not exert any significant changes in HO-1 on mRNA expressions. PCEE and silymarin significantly (P ≤ 0.01) decreased the levels of HO-1 on mRNA expression by 5.06-, 2.7-, and 1.6-fold in groups III, IV, and V, respectively.
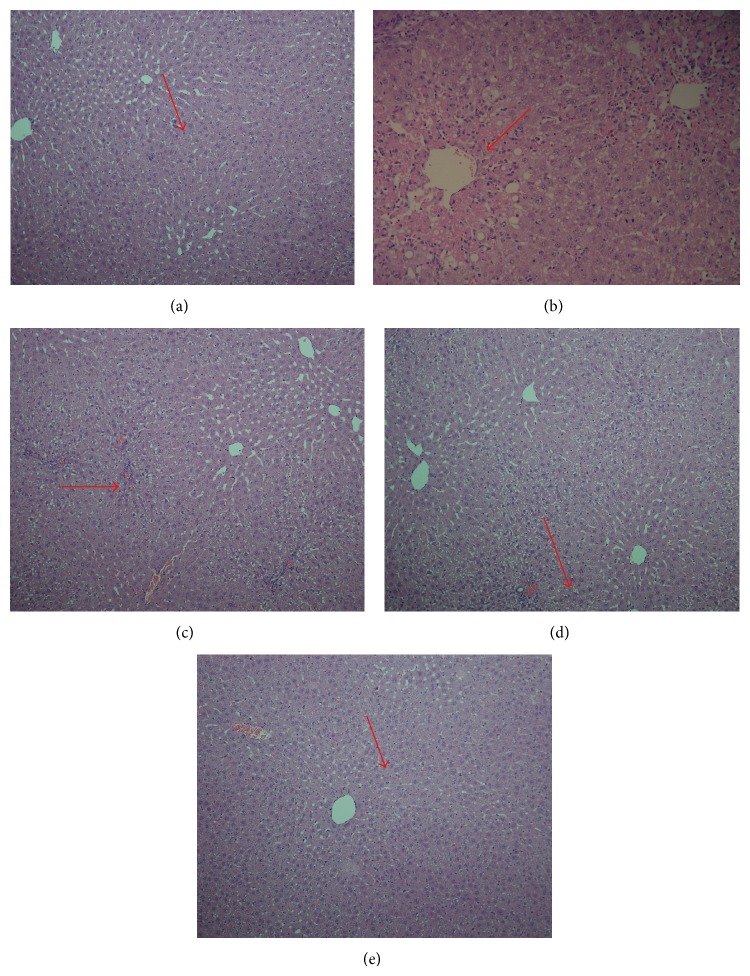
4.7. Histopathological Observations
The histological observations of liver tissues support the results obtained from serum enzyme assays. Liver section of normal control rat shows central vein surrounded by hepatic cord of cells while liver section of CCl4-treated rats shows massive fatty changes, focal central vein congestion, vacuolization, necrosis with inflammation, and loss of cellular boundaries. Liver section of rats treated with CCl4 and pretreated with PCEE 250 mg/kg shows mild central vein congestion, vocalization, and necrosis with sinusoidal dilatation. Liver sections of rats treated with CCl4 and 500 mg/kg of PCEE show absence of vocalization, inflammatory cells, and regeneration of hepatocytes around central vein but slight congestion in central vein and almost toward near normal liver architecture and possessing higher hepatoprotective action. The effect of silymarin was similar to that of PCEE (500 mg/kg) (Figures 3(a)–3(e)).
Figure 3.
Histopathology of liver tissues. (a) Liver section of normal control rat shows central vein surrounded by hepatic cord of cells (normal architecture), (b) liver section of CCl4-treated rats showing massive fatty changes, focal central vein congestion (indicated by arrow), ballooning formation, necrosis with inflammation, and loss of cellular boundaries, (c) liver section of rats treated with CCl4 and 250 mg/kg of PCEE showing mild central vein congestion (indicated by arrow), ballooning, and necrosis with sinusoidal dilatation, (d) liver section of rats treated with CCl4 and 500 mg/kg of PCEE showing absence of ballooning, inflammatory cells, and regeneration of hepatocytes around central vein toward near normal liver architecture but slight congestion in central vein (indicated by arrow), and (e) liver section of rats treated with CCl4 and 10 mg/kg of silymarin showing normal liver architecture.
4.8. In Vitro Antioxidant Activity of PCEE
The potential antioxidant activity of the PCEE was investigated on the basis of DPPH radical scavenging activity and of inhibition of linoleic acid oxidation. As demonstrated in Table 3, PCEE was able to reduce the stable free radical DPPH to the yellow-colored DPPH at high concentrations (500 and 1000 μg/mL). In addition to that, in the β-carotene/linoleic acid model system, the PCEE was also able to inhibit the discoloration of β-carotene at a concentration of 1000 μg/mL. The total antioxidant value was 79% (Table 3). The observed antioxidant activity was comparable to that of the positive control, rutin (Table 3).
Table 3.
In vitro free radical scavenging and antioxidant activity of Piper cubeba ethanolic extracts (PCEE).
| Plant species | Radical scavenging activity (%) | Total antioxidant activity (%) | ||||
|---|---|---|---|---|---|---|
| 10 | 50 | 100 | 500 | 1000 | 1000 (µg/mL) | |
| PCEE | 9.8 | 15.1 | 33.7 | 75.2 | 82.8 | 79.1 ± 5.26 |
| Ascorbic acid | 20.4 | 71.5 | 86.9 | 91.0 | 93.2 | — |
| Rutin | — | — | — | — | — | 92.5 ± 6.54 |
4.9. Phytochemical Screening
The preliminary qualitative screening of PCEE revealed the presence of volatile oils, terpenes, and flavonoids.
5. Discussion
The change in dietary habits and chemoprevention show considerable effective strategy against oxidative stress and are the main focus of area of research these days [33]. Various reports have shown that several mutagens and carcinogens cause generation of peroxide radicals, which play a major role in the emergence of cancer and other health disturbances [25, 34]. The current investigation was undertaken to evaluate the possible protective effect of PCEE against carbon tetrachloride induced hepatotoxicity and oxidative stress in rats. CCl4 is a known, reliable, and commonly used chemical to induce liver damage. The present study revealed that CCl4 induction in rats remarkably increased the level of SGPT, SGOT, GGT, and ALP. CCl4 causes acute hepatocyte injuries and altered membrane integrity and as a result enzymes in hepatocytes leak out [35]. However, after pretreatment with PCEE and silymarin, the pathological increases in SGOT, SGPT, γ-GGT, and ALP were significantly restored. These results indicate that PCEE and silymarin have the ability to protect against CCl4-induced hepatocyte injuries. The mechanism of CCl4-induced liver damage is known to be mediated through free radical reactions [10]. The metabolism of CCl4 toxicity lies in its biotransformation by the cytochrome P450 system to two reactive metabolites such as trichloromethyl (CCl3 +) free radicals and trichloromethylperoxy (CCl3OO∙) in the endoplasmic reticulum [36, 37] of the liver and initiated lipid peroxidation process [38].
CCl4 is reported to induce hepatic damage as a result of metabolic conversion of the radicals through lipid peroxidation and disturbance of the activities of the antioxidant enzymes [39] and induce oxidative stress and cause liver injury by the formation of free radicals [40]. Carbon tetrachloride, on the other hand, causes noticeable toxicity by enhancing liver lipid peroxidation (LPO), as found by increased concentrations of hepatic malondialdehyde (MDA) [41, 42]. Malondialdehyde, an end product of LPO, in liver tissue serves as an indicator of LPO, which is known to occur in hepatic toxicity due to the generation of reaction oxygen species (ROS) [42]. A significant decrease of MDA level was observed in rats treated with PCEE and silymarin. PCEE might be protecting the hepatocyte by impairing CCl4-mediated lipid peroxidation and resulting in the prevention of the generation of free radical derivatives [43]. The liver intoxication provoked by CCl4 causes a significant depletion of nonprotein sulfhydryl contents of the liver tissue which is an important indicator towards indicating the oxidative damage of liver. Depletion in NPSH level within living organisms causes tissue injury and further dysfunction [44]. However, pretreatment with PCEE significantly prevented the CCl4-induced decreased hepatic NPSH, indicating an antioxidant property of the extract in CCl4-induced liver toxicity. These findings show that Piper cubeba extract possesses the ability to scavenge reactive free radicals that diminish oxidative stress or damage of the liver tissue and provoke the activities of the hepatic antioxidant enzymes. AS1-SH1 acts as a nonenzymatic antioxidant, both intra- and extracellularly involved in the protection of normal cell integrity and function by redox and detoxification 15 reaction [45]. Furthermore, the current study manifested a pronounced diminution in liver tissue total protein (TP) level in CCl4 only treated rats, whereas TP level in the liver significantly elevated after the administration of PCEE and silymarin. Total protein level can also be used as one of the biomarkers to determine liver function [46]. This indicates that PCEE and silymarin induced hepatic cell proliferation which is sign for liver regeneration.
Catalase (CAT) is an enzymatic antioxidant widely distributed in all animal tissues, and the highest activity is found in the red cells and liver. Serum catalase (CAT) is the most sensitive enzymatic index in liver injury caused by reactive oxygen species (ROS) and oxidative stress [47, 48]. CAT is a hemoprotein which protects the cells from the accumulation of H2O2 [49]. Therefore, reduction in the activity of CAT may result in a number of deleterious effects due to the assimilation of superoxide radical and hydrogen peroxide. A higher dose (500 mg/kg) increases the level of CAT as produced by silymarin.
To investigate the underlying mechanism, we evaluated the effect of PCEE on the mRNA expression of certain proinflammatory cytokines related inflammation and proliferation. TNF-α, IL-6, and IL-10 as acute phase genes are considered to be special biomarkers for inflammation [50]. ROS upregulate NF-κB, which further induced proinflammatory cytokines, such as IL-1β, TNF-α, and IL-6 [51]. TNF-α and IL-6 are key mediators of inflammatory responses and control the expression of the inflammatory gene network. Therefore, the induction of TNF-α and IL-6 contributes significantly to the hepatic injury, which is associated with the upregulation of TNF-α and IL-6 gene expression that was observed in the CCl4 group. This result is therefore in accordance with previous studies [26]. Consequently, the induction of TNF-α and IL-6 contributed to the manifestation of the systemic inflammatory response and ultimately to the development of organ failure. The attenuation in level of inflammatory cytokines may explain the accelerated liver regeneration as observed in PCEE and silymarin administrated rat. The release of TNF-α, as a proinflammatory mediator in liver apoptosis, is also linked to cytotoxicity induced by CCl4 [52, 53].
Interleukin-10 (IL-10) is a major anti-inflammatory cytokine that potently inhibits production of proinflammatory mediators such as TNF-α and IL-12 [54]. Treatment of rats with PCEE and silymarin for one week prior to CCl4 administration increased the mRNA expression of IL-10 which adds more evidence of the anti-inflammatory effect of PCEE and silymarin in the acute phase response to CCl4. The upregulation of IL-10 by PCEE and silymarin indicates its ability to downregulate the inflammatory cytokine. Inflammatory liver involving oxidative stress initiates upregulation of HO-1 mRNA expression and increase in products of heme degradation pathway [55, 56]. Among these products, bilirubin and CO are the key mediators of inducible HO-1 mediated cytoprotection for the reason that they help restore intracellular homeostatic balance under oxidative stress conditions and help suppress inflammation through downregulation of proinflammatory mediators [55, 57–60]. The results of HO-1 on mRNA expression showed that treatment of CCl4, indicating the trend of increased HO-1 m RNA expression, while PCEE and silymarin pretreated CCl4 rats showed marked reduction in HO-1 on mRNA expression in dose dependent manner. This clearly indicates that PCEE and silymarin have cytoprotective role. The mRNA expressions of iNOS-2 were significantly increased by CCl4, which can be attributed to the reported CCl4-induced NO production [61, 62]. PCEE pretreatment has a significant reducing effect on iNOS-2 mRNA expression in CCl4-treated rats. PCEE and silymarin have mediated cytoprotective actions by suppressing iNOS and HO-1 mRNA expression.
The hepatoprotective effect of the PCEE was further accomplished by the histopathological examinations. PCEE at different dose levels offered hepatoprotection. PCEE 500 mg/kg exhibited similar results to silymarin. The preliminary qualitative analysis indicates the presence of essential oil, terpenoids, and flavonoids which are known antioxidants and anti-inflammatory agents [63, 64].
6. Conclusion
Findings of this study demonstrated that PCEE is effective in prevention of CCl4-induced hepatic damage in rats. Our results demonstrated that the treatment with PCEE significantly and dose dependently prevented drug induced increase in serum levels of hepatic enzymes. Furthermore, PCEE significantly reduced the lipid peroxidation in the liver tissue and restored activities of defense antioxidant enzymes NP-SH and CAT towards normal levels. The hepatoprotective effect of PCEE is attributed to downregulation of proinflammatory cytokines, for example, TNF-α and IL-6 mRNA expression as well as mRNA expression of iNOS and HO-1 gene, and upregulation of the IL-10. Histopathological studies have also shown that the PCEE and silymarin could prevent CCl4-induced hepatic damage in the liver.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the Research Group Project no. RGP-VPP-073.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Chopra R. N., Nayer S. L., Chopra I. C. Glossary of Indian Medicinal Plants. New Delhi, India: Council of Scientific & Industrial Research (India), NISCAIR; 1956. [Google Scholar]
- 2.Choi E.-M., Hwang J.-K. Investigations of anti-inflammatory and antinociceptive activities of Piper cubeba, Physalis angulata and Rosa hybrida . Journal of Ethnopharmacology. 2003;89(1):171–175. doi: 10.1016/S0378-8741(03)00280-0. [DOI] [PubMed] [Google Scholar]
- 3.Nahak G., Sahu R. K. Phytochemical evaluation and antioxidant activity of Piper cubeba and Piper nigrum. Journal of Applied Pharmaceutical Science. 2011;1(8):153–157. [Google Scholar]
- 4.Zaidi S. F. H., Yamada K., Kadowaki M., Usmanghani K., Sugiyama T. Bactericidal activity of medicinal plants, employed for the treatment of gastrointestinal ailments, against Helicobacter pylori. Journal of Ethnopharmacology. 2009;121(2):286–291. doi: 10.1016/j.jep.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Khan M., Siddiqui M. Antimicrobial activity of Piper fruits. Natural Product Radiance. 2007;6(2):111–113. [Google Scholar]
- 6.Ahmad Q. Z., Jahan N., Ahmad G. Nephroprotective effect of Kabab chini (Piper cubeba) in gentamycin-induced nephrotoxicity. Saudi Journal of Kidney Diseases and Transplantation. 2012;23(4):773–781. doi: 10.4103/1319-2442.98159. [DOI] [PubMed] [Google Scholar]
- 7.Gayasuddin M. M., Shakil S. S., Kavimani S. Effect of ethanolic extract of Piper cubeba Linn. fruits on activity of pioglitazone. International Journal of Pharmacy & Industrial Research. 2011;1:312–314. [Google Scholar]
- 8.Cauwels A., Brouckaert P. Survival of TNF toxicity: dependence on caspases and NO. Archives of Biochemistry and Biophysics. 2007;462(2):132–139. doi: 10.1016/j.abb.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Seitz H. K., Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biological Chemistry. 2006;387(4):349–360. doi: 10.1515/BC.2006.047. [DOI] [PubMed] [Google Scholar]
- 10.Recknagel R. O., Glende E. A., Jr., Dolak J. A., Waller R. L. Mechanisms of carbon tetrachloride toxicity. Pharmacology and Therapeutics. 1989;43(1):139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 11.Weber L. W. D., Boll M., Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Critical Reviews in Toxicology. 2003;33(2):105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 12.Bradley D. W., Maynard J. E., Emery G., Webster H. Transaminase activities in serum of long-term hemodialysis patients. Clinical Chemistry. 1972;18(11, article 1442) [PubMed] [Google Scholar]
- 13.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. The American Journal of Clinical Pathology. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 14.Persijn J. P., van der Slik W. A new method for the determination of γ glutamyltransferase in serum. Journal of Clinical Chemistry and Clinical Biochemistry. 1976;14(9):421–427. doi: 10.1515/cclm.1976.14.1-12.421. [DOI] [PubMed] [Google Scholar]
- 15.Jendrassik L., Groff P. Simplified photometric method for determination of blood bilirubin. Biochemical Journal. 1938;297:81–89. [Google Scholar]
- 16.Lum G., Gambino S. R. A comparison of serum versus heparinized plasma for routine chemistry tests. The American Journal of Clinical Pathology. 1974;61(1):108–113. doi: 10.1093/ajcp/61.1.108. [DOI] [PubMed] [Google Scholar]
- 17.Utley H. G., Bernheim F., Hochstein P. Effect of sulfhydryl reagents on lipid peroxidation in microsome. Archives of Biochemistry and Biophysics. 1967;118:29–32. doi: 10.1016/0003-9861(67)90273-1. [DOI] [Google Scholar]
- 18.Konukoğlu D., Akçay T., Dinçer Y., Hatemi H. The susceptibility of red blood cells to autoxidation in type 2 diabetic patients with angiopathy. Metabolism: Clinical and Experimental. 1999;48(12):1481–1484. doi: 10.1016/S0026-0495(99)90233-0. [DOI] [PubMed] [Google Scholar]
- 19.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 20.Sedlak J., Lindsay R. H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Analytical Biochemistry. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 21.Aebi H. Catalase. Weinheim, Germany: Academic Press; 1974. [Google Scholar]
- 22.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry. 1987;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 23.Pfaffl M. W., Horgan G. W., Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Research. 2002;30(9, article e36) doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfaffl M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29(9, article e45) doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radical Biology and Medicine. 1990;8(6):583–599. doi: 10.1016/0891-5849(90)90156-D. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim Z. S., Ishizuka M., Soliman M., et al. Protection by Nigella sativa against carbon tetrachloride-induced downregulation of hepatic cytochrome P450 isozymes in rats. Japanese Journal of Veterinary Research. 2008;56(3):119–128. [PubMed] [Google Scholar]
- 27.Siegling A., Lehmann M., Platzer C., Emmrich F., Volk H. D. A novel multispecific competitor fragment for quantitative PCR analysis of cytokine gene expression in rats. Journal of Immunological Methods. 1994;177(1-2):23–28. doi: 10.1016/0022-1759(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 28.Grilli A., De Lutiis M. A., Patruno A., et al. Inducible nitric oxide synthase and heme oxygenase-1 in rat heart: direct effect of chronic exposure to hypoxia. Annals of Clinical and Laboratory Science. 2003;33(2):208–215. [PubMed] [Google Scholar]
- 29.Maayah Z. H., Ansari M. A., El Gendy M. A., Al-Arifi M. N., Korashy H. M. Development of cardiac hypertrophy by sunitinib in vivo and in vitro rat cardiomyocytes is influenced by the aryl hydrocarbon receptor signaling pathway. Archives of Toxicology. 2014;88(3):725–738. doi: 10.1007/s00204-013-1159-5. [DOI] [PubMed] [Google Scholar]
- 30.Brand-Williams W., Cuvelier M. E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Science and Technology. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 31.Mothana R. A. A. Anti-inflammatory, antinociceptive and antioxidant activities of the endemic Soqotraen Boswellia elongata Balf. f. and Jatropha unicostata Balf. f. in different experimental models. Food and Chemical Toxicology. 2011;49(10):2594–2599. doi: 10.1016/j.fct.2011.06.079. [DOI] [PubMed] [Google Scholar]
- 32.Wagner H., Bladt S. Plants Drug Analysis: A Thin Layer Chromatography Atlas. Berlin, Germany: Springer; 1996. [Google Scholar]
- 33.Lee B. M., Park K.-K. Beneficial and adverse effects of chemopreventive agents. Mutation Research. 2003;523-524:265–278. doi: 10.1016/S0027-5107(02)00342-1. [DOI] [PubMed] [Google Scholar]
- 34.Aleynik S. I., Leo M. A., Ma X., Aleynik M. K., Lieber C. S. Polyenylphosphatidylcholine prevents carbon tetrachloride-induced lipid peroxidation while it attenuates liver fibrosis. Journal of Hepatology. 1997;27(3):554–561. doi: 10.1016/S0168-8278(97)80361-3. [DOI] [PubMed] [Google Scholar]
- 35.Hu Y. Y., Liu C. H., Wang R. P., Liu C., Liu P., Zhu D. Y. Protective actions of salvianolic acid A on hepatocyte injured by peroxidation in vitro. World Journal of Gastroenterology. 2000;6(3):402–404. doi: 10.3748/wjg.v6.i3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Britton R. S., Bacon B. R. Role of free radicals in liver diseases and hepatic fibrosis. Hepato-Gastroenterology. 1994;41(4):343–348. [PubMed] [Google Scholar]
- 37.Mohideen S., Ilavarasan R., Sasikala E., Kumaran R. T. Hepatoprotective activity of Nigella sativa Linn. Indian Journal of Pharmaceutical Sciences. 2003;65(5):550–551. [Google Scholar]
- 38.Badarinath A. V., Rao K. M., Chetty C. M. S., Ramkanth S., Rajan T. V. S., Gnanaprakash K. A review on in-vitro antioxidant methods: comparisions, correlations and considerations. International Journal of PharmTech Research. 2010;2(2):1276–1285. [Google Scholar]
- 39.Adesanoye O. A., Farombi E. O. Hepatoprotective effects of Vernonia amygdalina (astereaceae) in rats treated with carbon tetrachloride. Experimental and Toxicologic Pathology. 2010;62(2):197–206. doi: 10.1016/j.etp.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Manna P., Sinha M., Sil P. C. Aqueous extract of Terminalia arjuna prevents carbon tetrachloride induced hepatic and renal disorders. BMC Complementary and Alternative Medicine. 2006;6, article 33 doi: 10.1186/1472-6882-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poli G. Liver damage due to free radicals. British Medical Bulletin. 1993;49(3):604–620. doi: 10.1093/oxfordjournals.bmb.a072634. [DOI] [PubMed] [Google Scholar]
- 42.Dalton S. R., Lee S. M. L., King R. N., et al. Carbon tetrachloride-induced liver damage in asialoglycoprotein receptor-deficient mice. Biochemical Pharmacology. 2009;77(7):1283–1290. doi: 10.1016/j.bcp.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin H.-M., Tseng H.-C., Wang C.-J., Lin J.-J., Lo C.-W., Chou F.-P. Hepatoprotective effects of Solanum nigrum Linn extract against CCl4-iduced oxidative damage in rats. Chemico-Biological Interactions. 2008;171(3):283–293. doi: 10.1016/j.cbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Al-Yahya M., Mothana R., Al-Said M., et al. Attenuation of CCl4-induced oxidative stress and hepatonephrotoxicity by Saudi Sidr honey in rats. Evidence-Based Complementary and Alternative Medicine. 2013;2013:10. doi: 10.1155/2013/569037.569037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahn J., Grün I. U., Mustapha A. Effects of plant extracts on microbial growth, color change, and lipid oxidation in cooked beef. Food Microbiology. 2007;24(1):7–14. doi: 10.1016/j.fm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Irshaid F. I., Mansi K., Bani-Khaled A., Aburjia T. Hepatoprotetive, cardioprotective and nephroprotective actions of essential oil extract of Artemisia sieberi in alloxan induced diabetic rats. Iranian Journal of Pharmaceutical Research. 2012;11(4):1227–1234. [PMC free article] [PubMed] [Google Scholar]
- 47.Sanmugapriya E., Venkataraman S. Studies on hepatoprotective and antioxidant actions of Strychnos potatorum Linn. seeds on CCl4-induced acute hepatic injury in experimental rats. Journal of Ethnopharmacology. 2006;105(1-2):154–160. doi: 10.1016/j.jep.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 48.Chance B., Greenstein D. S., Roughton F. J. W. The mechanism of catalase action. I. Steady-state analysis. Archives of Biochemistry and Biophysics. 1952;37(2):301–321. doi: 10.1016/0003-9861(52)90194-X. [DOI] [PubMed] [Google Scholar]
- 49.Oh S. I., Kim C.-I., Chun H. J., Lee M. S., Park S. C. Glutathione recycling is attenuated by acute ethanol feeding in rat liver. Journal of Korean Medical Science. 1997;12(4):316–321. doi: 10.3346/jkms.1997.12.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reyes-Gordillo K., Segovia J., Shibayama M., Vergara P., Moreno M. G., Muriel P. Curcumin protects against acute liver damage in the rat by inhibiting NF-κB, proinflammatory cytokines production and oxidative stress. Biochimica et Biophysica Acta—General Subjects. 2007;1770(6):989–996. doi: 10.1016/j.bbagen.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Lim Y., Levy M., Bray T. M. Dietary zinc alters early inflammatory responses during cutaneous wound healing in weanling CD-1 mice. Journal of Nutrition. 2004;134(4):811–816. doi: 10.1093/jn/134.4.811. [DOI] [PubMed] [Google Scholar]
- 52.Wu Y.-L., Lian L.-H., Wan Y., Nan J.-X. Baicalein inhibits nuclear factor-κB and apoptosis via c-FLIP and MAPK in d-GalN/LPS induced acute liver failure in murine models. Chemico-Biological Interactions. 2010;188(3):526–534. doi: 10.1016/j.cbi.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Diao Y., Zhao X.-F., Lin J.-S., Wang Q.-Z., Xu R.-A. Protection of the liver against CCl4-induced injury by intramuscular electrotransfer of a kallistatin-encoding plasmid. World Journal of Gastroenterology. 2011;17(1):111–117. doi: 10.3748/wjg.v17.i1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cassatella M. A., Meda L., Bonora S., Ceska M., Constantin G. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1β in mediating the production of IL-8 triggered by lipopolysaccharide. Journal of Experimental Medicine. 1993;178(6):2207–2211. doi: 10.1084/jem.178.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ryter S. W., Choi A. M. K. Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy. The American Journal of Respiratory Cell and Molecular Biology. 2009;41(3):251–260. doi: 10.1165/rcmb.2009-0170TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vardanian A. J., Busuttil R. W., Kupiec-Weglinski J. W. Molecular mediators of liver ischemia and reperfusion injury: a brief review. Molecular Medicine. 2008;14(5-6):337–345. doi: 10.2119/2007-00134.Vardanian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lekić N., Canová N. K., Hořínek A., Farghali H. The involvement of heme oxygenase 1 but not nitric oxide synthase 2 in a hepatoprotective action of quercetin in lipopolysaccharide-induced hepatotoxicity of d-galactosamine sensitized rats. Fitoterapia. 2013;87(1):20–26. doi: 10.1016/j.fitote.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 58.Gomes A. S., Gadelha G. G., Lima S. J., et al. Gastroprotective effect of heme-oxygenase 1/biliverdin/CO pathway in ethanol-induced gastric damage in mice. European Journal of Pharmacology. 2010;642(1–3):140–145. doi: 10.1016/j.ejphar.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 59.Paine A., Eiz-Vesper B., Blasczyk R., Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochemical Pharmacology. 2010;80(12):1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 60.Wu L., Wang R. Carbon monoxide: Endogenous production, physiological functions, and pharmacological applications. Pharmacological Reviews. 2005;57(4):585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 61.Chakravortty D., Kato Y., Sugiyama T., Koide N., Yoshida T., Yokochi T. Inhibition of caspase 3 abrogates lipopolysaccharide-induced nitric oxide production by preventing activation of NF-κB and c-jun NH2-terminal kinase/stress-activated protein kinase in RAW 264.7 murine macrophage cells. Infection and Immunity. 2001;69(3):1315–1321. doi: 10.1128/IAI.69.3.1315-1321.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mu M. M., Chakravortty D., Sugiyama T., et al. The inhibitory action of quercetin on lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophage cells. Journal of Endotoxin Research. 2001;7(6):431–438. doi: 10.1179/096805101101533034. [DOI] [PubMed] [Google Scholar]
- 63.Saleh M. A., Clark S., Woodard B., Deolu-Sobogun S. A. Antioxidant and free radical scavenging activities of essential oils. Ethnicity & disease. 2010;20(supplement 1):78–82. [PubMed] [Google Scholar]
- 64.Miguel M. G. Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules. 2010;15(12):9252–9287. doi: 10.3390/molecules15129252. [DOI] [PMC free article] [PubMed] [Google Scholar]