Figure 1.
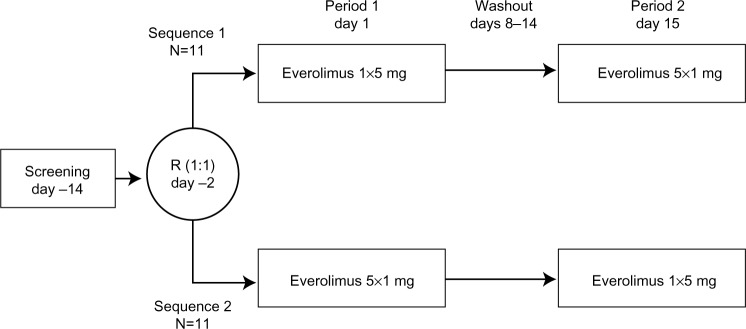
Study design.
Notes: End of pharmacokinetic sampling occurred at days 7 and 21. End of study evaluation was performed 14 (±2) days after everolimus administration in the second time period.
Abbreviation: R, randomization.

Study design.
Notes: End of pharmacokinetic sampling occurred at days 7 and 21. End of study evaluation was performed 14 (±2) days after everolimus administration in the second time period.
Abbreviation: R, randomization.